2. Expanded Porphyrin
2-1. N-Confused Sapphyrin
The first expanded porphyrins with a confused moiety are N-confused dithia- and diselenasapphyrin (NCS2S, NCSe2S ) (JACS 2001). The inverted structure of NCS2S is elucidated by X-ray analysis.
2-2. N-Confused Pentaphyrin
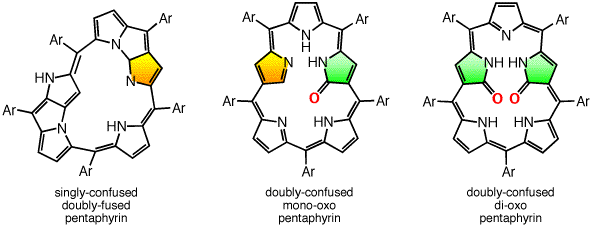
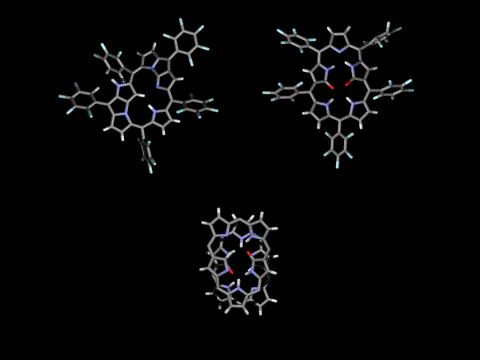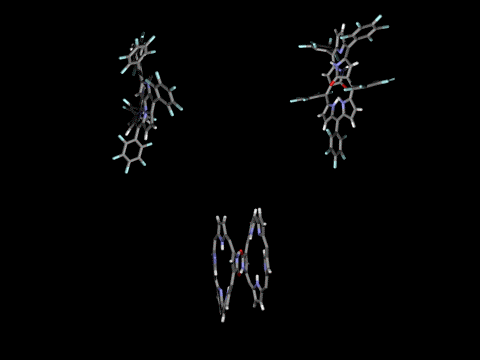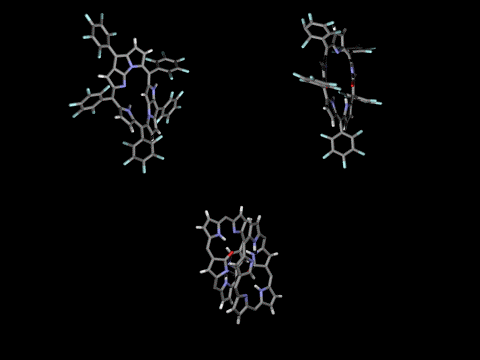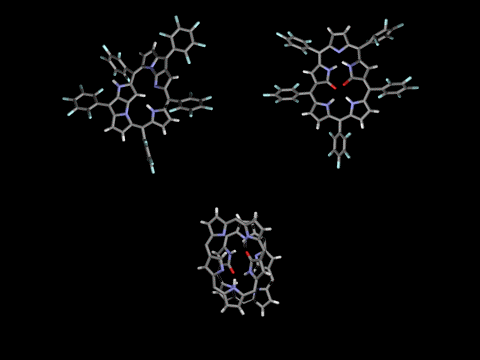
Singly- and doubly N-confused pentaphyrins (NCP5, N2CP5) were tried to synthesize. However, doubly-fused pentaphyrin (NF2P5) was obtained from the NCP5 synthesis (ACIE 2004) and mono-oxo and di-oxo doubly N-confused pentaphyrins (N2COP5, N2CO2P5 ) were formed in the N2CO2P5 synthesis, depending on the strength of oxidants in the cyclization step. In the solid state, N2COP5 forms dimeric structure by the intermolecular hydrogen bonding between amide groups, whereas the dioxo derivative, N2CO2P5, exists in a monomeric form owing to the intramolecular hydrogen bonding (ACIE 2004).
2-3. Doubly N-Confused Hexaphyrin
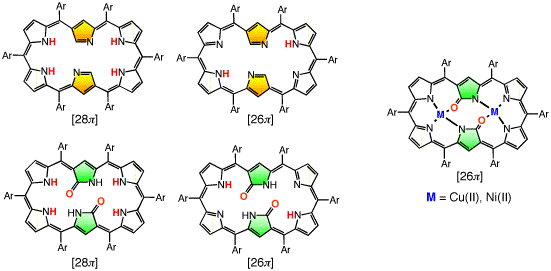
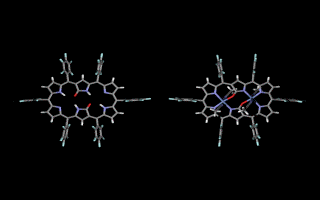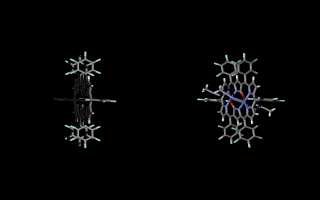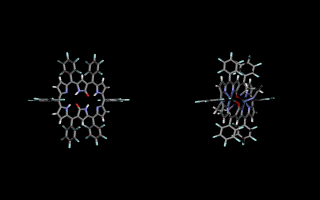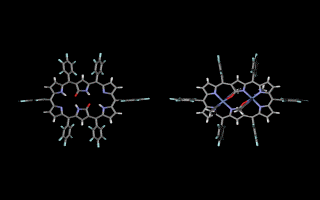
Doubly N-confused hexaphyrin (N2CH) was synthesized from a [3+3] couping of confused tripyrrane. N2CH adopts two oxdation states ([26π] and [28π]) just like normal hexaphyrin, and the corresponding oxo-substituted derivatives (amide-N2CH) also does. Amide-N2CH is highly planar because of the intramolecular hydrogen bonding between the two amide moieties, and it captures two metal ions in the larger core than that of normal porphyrin. Bis-Cu(II), Co(II), Ni(II), Zn(II), Mn(III), Fe(III) amide-N2CH complexes were obtained and the structures were determined by X-ray analyses (JACS 2003, CEJ 2006). Additionally, amide-N2CH metal complexes were found to exhibit ultrafast optical responses (Mol. Cryst. Liq. Cryst. 2006, JPC. B 2006).
2-4. Doubly and Quadruply N-Confused Octaphyrin
Syntheses are now under going.
Back to Content









