儘田 正史

| 略歴 | |
|---|---|
| 2003.3 | 国立群馬工業高等専門学校 物質工学科 卒業 |
| 2005.3 | 国立群馬工業高等専門学校専攻科 環境工学専攻 修了 |
| 2007.3 | 東京工業大学大学院 総合理工学研究科 博士前期課程 物質電子化学専攻 修了 |
| 2010.3 | 東京工業大学大学院 総合理工学研究科 博士後期課程 物質電子化学専攻 修了 |
| 2009.4-2010.3 | 日本学術振興会 特別研究員 |
| 2009.9-2010.12 | University of Washington 訪問研究員 |
| 2010.4-2011.9 | Bowling Green State University(ボーリンググリーン州立大学) 博士研究員 |
| 2015.8–2015.12 | Johannes Kepler Universitat訪問研究員 |
| 2011.10–2016.1 | 山形大学大学院理工学研究科 助教 |
| 2016.2–2018.6 | 九州大学最先端有機光エレクトロニクス研究センター 助教 |
| 2018.6–2019-3 | 九州大学分子システムデバイス国際リーダー教育センター 助教 |
| 2019.4–現在 | 九州大学大学院工学研究院応用化学部門 助教 |
| 学位 | |
| 博士(理学) | |
| 所属学会 | |
| 日本化学会、有機合成化学協会、応用物理学会、有機EL討論会 | |
| 研究分野 | |
| 構造有機化学、有機合成化学、有機電子デバイス | |
| 受賞歴 | |
| 令和4年度科学技術分野の文部科学大臣表彰 若手科学者賞 | |
| 有機EL討論会 講演奨励賞受賞、他 | |
| 趣味 | |
| 料理、お酒、ボード、収集、メガネ、ダーツ、ダイビング | |
| 健康状態 | |
| 猫アレルギー | |
研究成果
雑誌カバー・扉絵
論文発表
[72] Masashi Mamada*, Satoshi Maedera, Susumu Oda, Thanh Ba Nguyen, Hajime Nakanotani, Takuji Hatakeyama, Chihaya Adachi*
“Very low lasing threshold of DABNA derivatives with DFB structures”
Mater. Chem. Front., 2022, in press.

[71] Monirul Hasan, Atul Shukla, Masashi Mamada, Chihaya Adachi, Shih-Chun Lo, Ebinazar B. Namdas*
“Correlating exciton dynamics of thermally activated delayed-fluorescence emitters to efficiency roll-off in OLEDs”
Phys. Rev. Applied, 2022, 2022, 18, 054082.
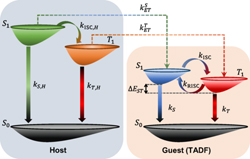
[70] Masashi Mamada*, Chihaya Adachi*
“Unexpected role of hole and electron blocking interlayers controlling charge carrier injection and transport in TADF based blue organic light-emitting diodes”
Appl. Phys. Lett., 2022, 121, 131103.
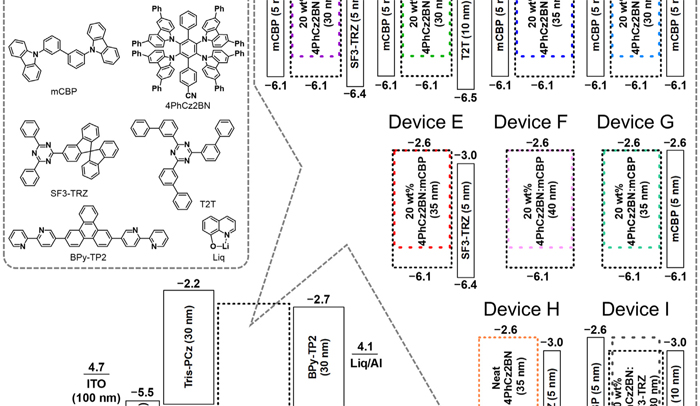
[69] Chin-Yiu Chan*, Yi-Ting Lee, Masashi Mamada, Kenichi Goushi, Youichi Tsuchiya, Hajime Nakanotani, Chihaya Adachi*
“Carbazole-2-carbonitrile as an acceptor in deep-blue thermally activated delayed fluorescence emitters for narrowing charge-transfer emissions”
Chem. Sci., 2022, 13, 7821–7828.
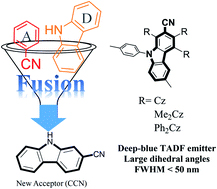
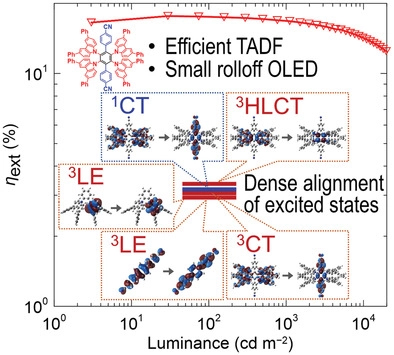
[68] Yi-Ting Lee, Chin-Yiu Chan*, Masaki Tanaka, Masashi Mamada, Kenichi Goushi, Xun Tang, Youichi Tsuchiya, Hajime Nakanotani, Chihaya Adachi*
“Tailor-made Multi-Resonance Terminal Emitters Towards Narrowband, High-Efficiency, and Stable Hyperfluorescence Organic Light-Emitting Diodes”
Adv. Opt. Mater., 2022, 2200682.
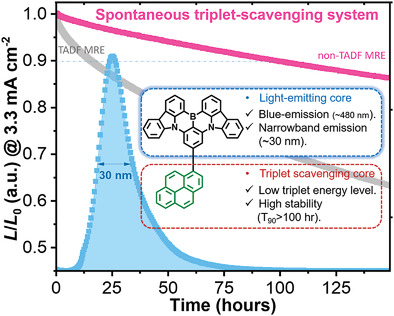
[67] Masashi Mamada*, Hiroshi Katagiri, Chin-Yiu Chan, Yi-Ting Lee, Kenichi Goushi, Hajime Nakanotani, Takuji Hatakeyama, Chihaya Adachi*
“Highly Efficient Deep-Blue Organic Light-Emitting Diodes Based on Rational Molecular Design and Device Engineering”
Adv. Funct. Mater., 2022, 2204352.
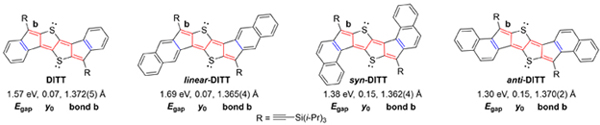
[66] Tanguy Jousselin-Oba, Masashi Mamada, Karen Wright, Jérome Marrot, Chihaya Adachi, Abderrahim Yassar, Michel Frigoli*
“Synthesis, Crystal Structure, Tropicity and Charge Transport Properties of Diindenothienothiophene Derivatives”
J. Mater. Chem. C, 2022, 10, 7717–7723.
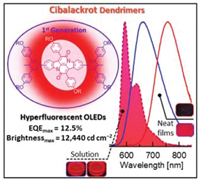
[65] Nicholle R. Wallwork, Masashi Mamada*, Angus B. Keto, Sarah K. M. McGregor, Atul Shukla, Chihaya Adachi, Elizabeth Krenske, Ebinazar B. Namdas*, Shih-Chun Lo*
“Cibalackrot Dendrimers for Hyperfluorescent Organic Light-Emitting Diodes”
Macromol. Rapid Commun., 2022, 2200118.
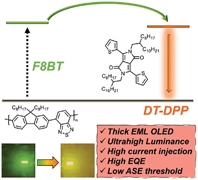
[64] Atul Shukla*, Volter Entoma, Sarah K. M. McGregor, Monirul Hasan, Masashi Mamada, Evan G. Moore, Chihaya Adachi, Shih-Chun Lo*, Ebinazar B. Namdas*
“Low Light Amplification Threshold and Reduced Efficiency Roll-Off in Thick Emissive Layer OLEDs from a Diketopyrrolopyrrole Derivative”
Macromol. Rapid Commun., 2022, 2200115.
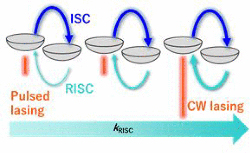
[63] Ayano Abe, Kenichi Goushi*, Atula S. D. Sandanayaka, Ryutaro Komatsu, Takashi Fujihara, Masashi Mamada, Chihaya Adachi*
“Numerical Study of Triplet Dynamics in Organic Semiconductors Aimed for the Active Utilization of Triplets by TADF under Continuous-Wave Lasing”
J. Phys. Chem. Lett., 2022, 13, 1323–1329.
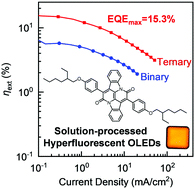
[62] Nicholle R. Wallwork, Masashi Mamada*, Atul Shukla, Sarah K. M. McGregor, Chihaya Adachi*, Ebinazar Namdas*, Shih-Chun Lo*
“High-Performance Solution Processed Red Hyperfluorescent OLEDs Based on Cibalackrot”
J. Mater. Chem. C, 2022, 10, 4767–4774.
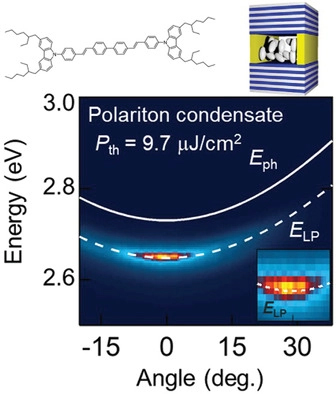
[61] Tomohiro Ishii, Kiyoshi Miyata, Masashi Mamada, Fatima Bencheikh, Fabrice Mathevet, Ken Onda, Stéphane Kéna-Cohen, Chihaya Adachi*
“Low-threshold exciton-polariton condensation via fast polariton relaxation in organic microcavities”
Adv. Opt. Mater., 2022, 10, 2102034.
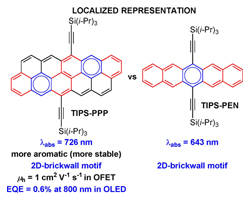
[60] Tanguy Jousselin-Oba, Masashi Mamada*, Karen Wright, Jérome Marrot, Chihaya Adachi, Abderrahim Yassar, Michel Frigoli*
“Synthesis, Aromaticity and Application of peri-PentacenoPentacene: Localized Representation of Benzenoid Aromatic Compounds”
Angew. Chem. Int. Ed., 2022, 61, e202112794.
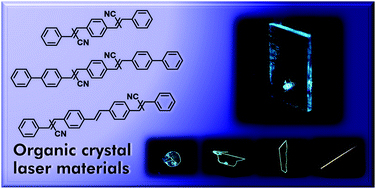
[59] Masashi Mamada*, Hajime Nakanotani, Chihaya Adachi*
“Amplified spontaneous emission from oligo(p-phenylenevinylene) derivatives”
Mater. Adv., 2021, 2, 3906–3914.
[58] Yi-Ting Lee, Chin-Yiu Chan, Masaki Tanaka, Masashi Mamada, Umamahesh Balijapalli, Youichi Tsuchiya, Hajime Nakanotani, Takuji Hatakeyama, Chihaya Adachi*
“Investigating HOMO Energy Levels of Terminal Emitters for Realizing High-Brightness and Stable TADF-Assisted Fluorescence Organic Light-Emitting Diodes”
Adv. Electron. Mater., 2021, 7, 2001090.
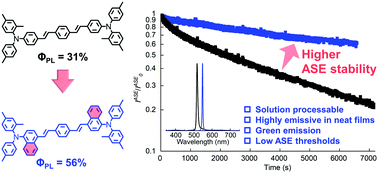
[57] Yuya Oyama, Masashi Mamada*, Akihiro Kondo*, Chihaya Adachi*
“Advantages of naphthalene as building block for organic solid state laser dyes: Smaller energy gaps and enhanced stability”
J. Mater. Chem. C, 2021, 9, 4112–4118.
Selected as the outside front cover picture [image]
[56] Xuelong Liu, Chin-Yiu Chan*, Fabrice Mathevet, Masashi Mamada, Youichi Tsuchiya, Yi-Ting Lee, Hajime Nakanotani, Shinichiro Kobayashi, Masayuki Shiochi, Chihaya Adachi*
“Isotope Effect of Host Material on Device Stability of Thermally Activated Delayed Fluorescence Organic Light-Emitting Diodes”
Small Sci., 2021, 1, 2000057.
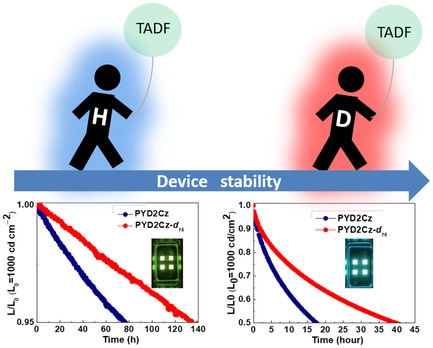
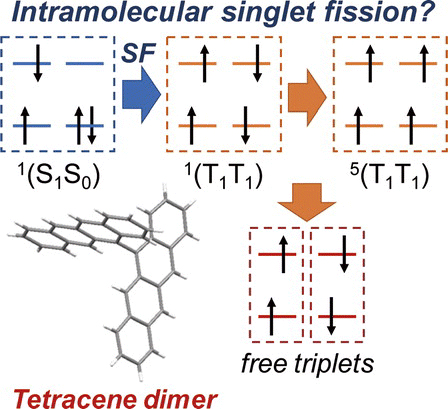
[55] Masashi Mamada*, Kenichi Goushi*, Ryota Nakamura, Hironori Kaji, Chihaya Adachi*
“Synthesis and Characterization of 5,5′-Bitetracene”
Chemistry Letters, 2021, 50, 800–803.
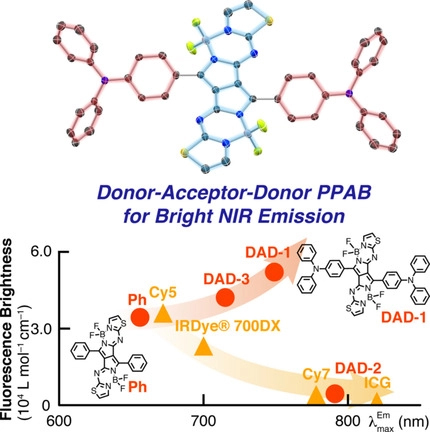
[54] Yuto Kage, Seongsoo Kang, Shigeki Mori, Masashi Mamada*, Chihaya Adachi, Dongho Kim*, Hiroyuki Furuta*, Soji Shimizu*
“An Electron-Accepting aza-BODIPY-Based Donor–Acceptor–Donor Architecture for Bright NIR Emission”
Chem. Eur. J., 2021, 27, 5259–5267.
Selected as a Very Important Paper
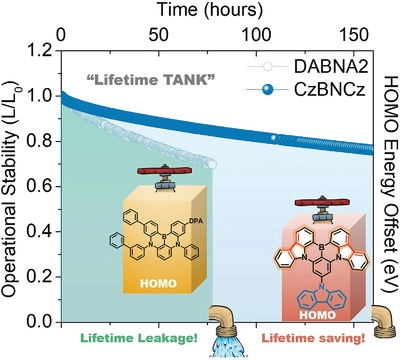
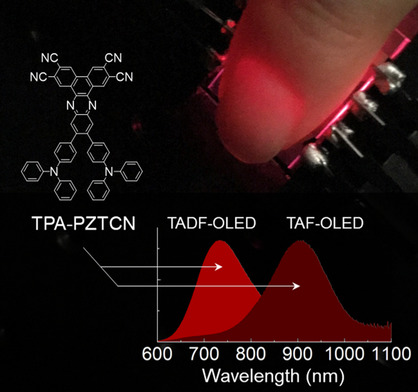
[53] Umamahesh Balijapalli, Ryo Nagata, Nishiki Yamada, Hajime Nakanotani*, Masaki Tanaka, Anthony D’Aléo, Virginie Placide, Masashi Mamada, Youichi Tsuchiya*, Chihaya Adachi*
“Highly Efficient Near-Infrared Electrofluorescence from a Thermally-Activated Delayed Fluorescence Molecule”
Angew. Chem. Int. Ed., 2021, 60, 8477–8482.
Selected as a Hot Paper
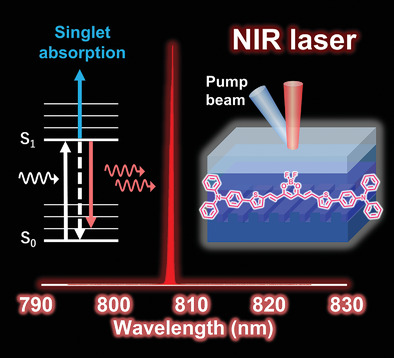
[52] Reiko Aoki, Ryutaro Komatsu, Kenichi Goushi, Masashi Mamada*, Soo Young Ko, Jeong Weon Wu, Virginie Placide, Anthony D’Aléo*, Chihaya Adachi*
“Realizing Near-Infrared Laser Dyes through a Shift in Excited-State Absorption”
Advanced Optical Materials, 2021, 9, 2001947.
Selected as the outside front cover picture [image]
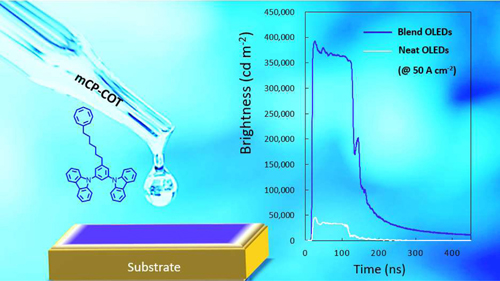
[51] Van Mai, Viqar Ahmad, Masashi Mamada, Toshiya Fukunaga, Atul Shukla, Jan Sobus, Gowri Krishnan, Evan Moore, Gunther Andersson, Chihaya Adachi*, Ebinazar Namdas*, Shih-Chun Lo
“Solid cyclooctatetraene-based triplet quencher demonstrating excellent suppression of singlet–triplet annihilation in optical and electrical excitation”
Nature Communications, 2020, 11, 5623.
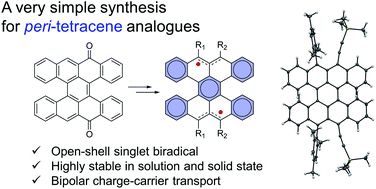
[50] Masashi Mamada*, Ryota Nakamura, Chihaya Adachi*
“Synthesis, crystal structure and charge transport characteristics of stable peri-tetracene analogues”
Chemical Science, 2021, 12, 552–558.
Selected as the inside front cover picture [image]
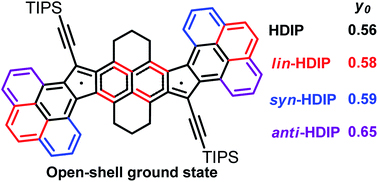
[49] Tanguy Jousselin-Oba, Masashi Mamada*, Atsushi Okazawa, Jérome Marrot, Takayuki Ishida, Chihaya Adachi, Abderrahim Yassar, Michel Frigoli*
“Modulating the ground state, stability and charge transport in OFETs of biradicaloid Hexahydro-diindenopyrene derivatives and a proposed method to estimate the biradical character”
Chemical Science, 2020, 11, 12194–12205.
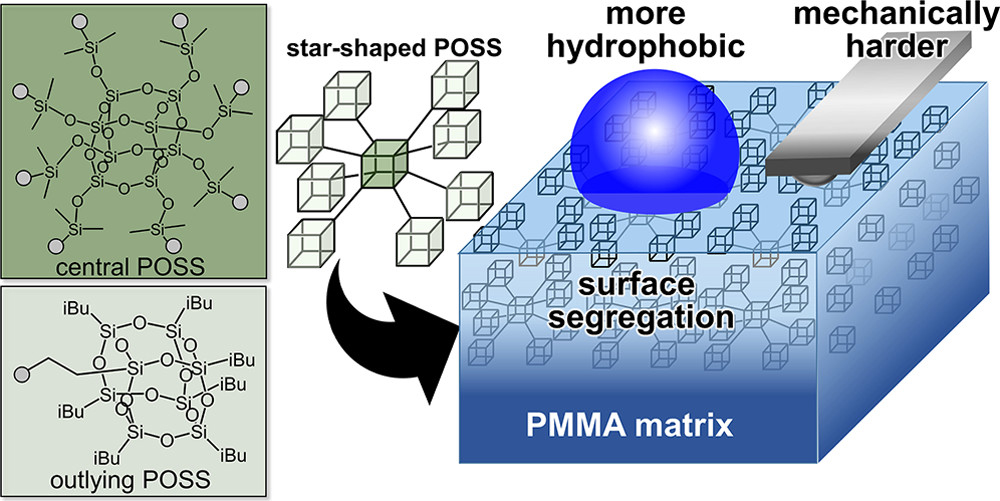
[48] Kentaro Yamamoto, Daisuke Kawaguchi*, Tatsuki Abe, Takeshi Komino, Masashi Mamada, Taizo Kabe, Chihaya Adachi, Kensuke Naka, Keiji Tanaka*
“Surface Segregation of Star-Shaped Polyhedral Oligomeric Silsesquioxane in Polymer Matrix”
Langmuir, 2020, 36, 9960–9966.
[47] Michel Frigoli*, Tanguy Jousselin-Oba, Masashi Mamada, Jérôme Marrot, Agnese Zangarelli, Danilo Pannacci, Chihaya Adachi, Fausto Ortica*
“Synthesis and photochromic behaviour of a series of benzopyrans bearing an N-phenyl-carbazole moiety: photochromism control by the steric effect”
Photochem. Photobiol. Sci., 2020, 19, 1344–1355.
[46] Viqar Ahmad, Jan Sobus, Fatima Bencheikh, Masashi Mamada, Chihaya Adachi, Shih-Chun Lo*, Ebinazar B. Namdas*
“High EQE and high brightness solution-processed TADF light-emitting transistors and OLEDs”
Adv. Opt. Mater., 2020, 202000554.
[45] Monirul Hasan, Atul Shukla, Viqar Ahmad, Jan Sobus, Fatima Bencheikh, Sarah M. K. McGregor, Masashi Mamada, Chihaya Adachi*, Shih-Chun Lo*, Ebinazar B. Namdas*
“Exciton-exciton annihilation in thermally activated delayed fluorescence emitter”
Adv. Funct. Mater., 2020, 30, 2000580.
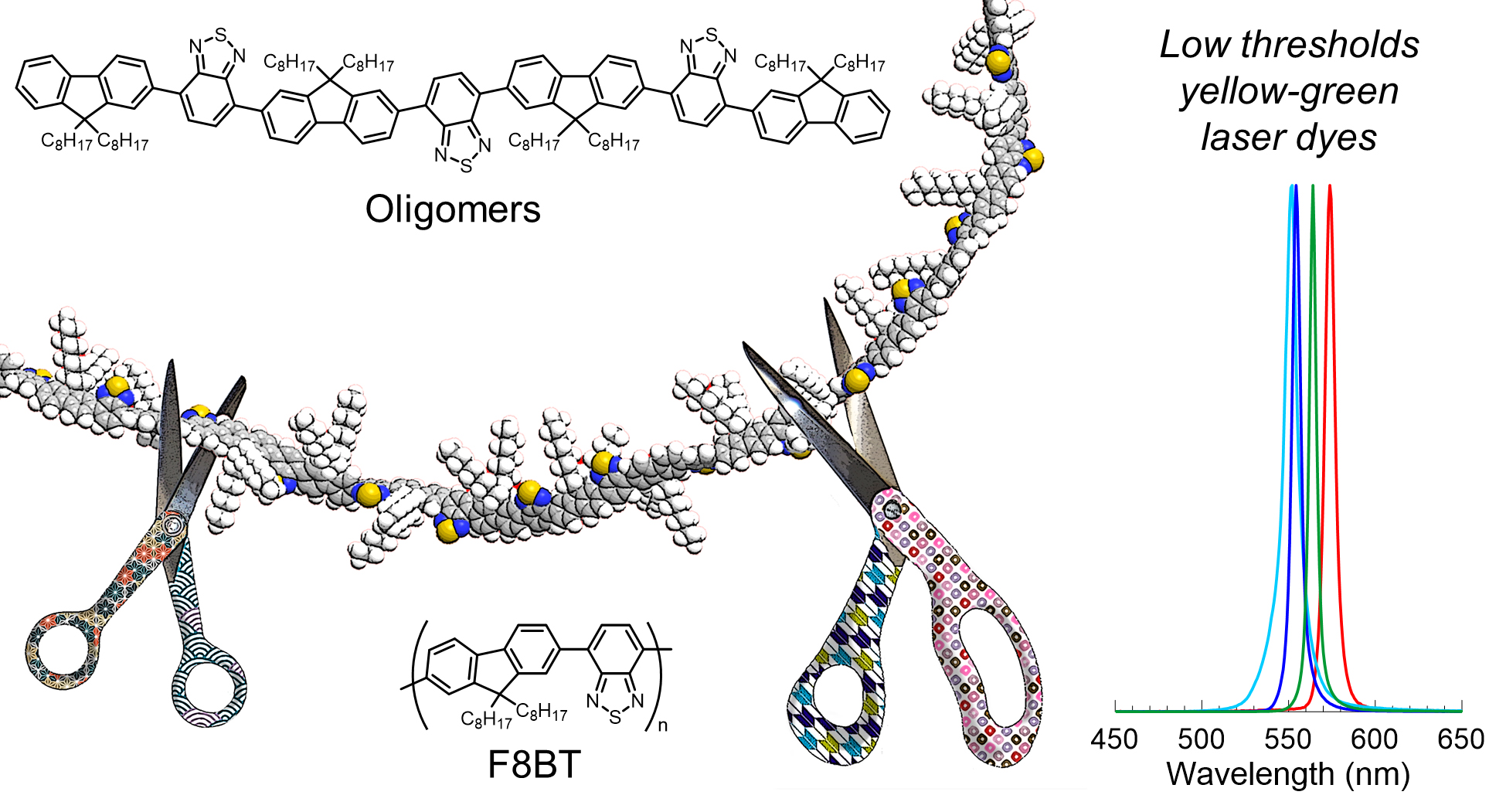
[44] Masashi Mamada*, Ryutaro Komatsu, Chihaya Adachi*
“F8BT Oligomers for Organic Solid-State Lasers”
ACS Appl. Mater. Interfaces, 2020, 12, 28383–28391.
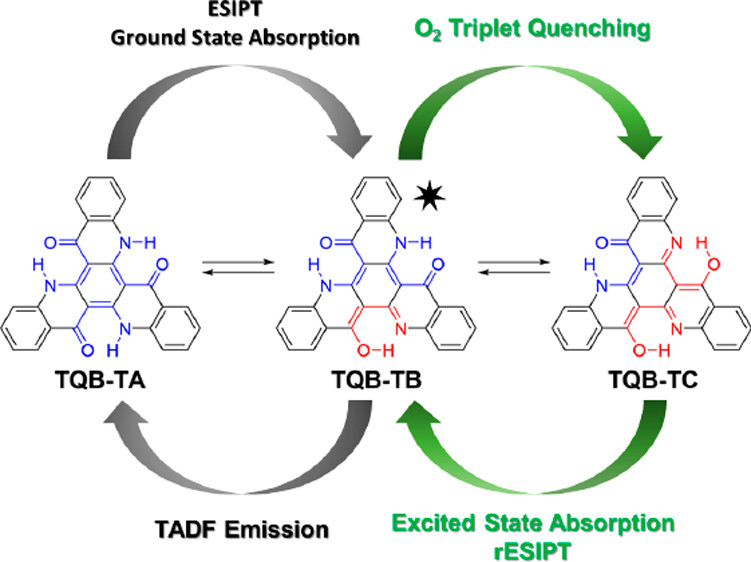
[43] Yun Long, Masashi Mamada, Chunyong Li, Paloma Lays dos Santos, Marco Colella, Andrew Danos, Chihaya Adachi, Andrew P. Monkman*
“Excited State Dynamics of Thermally Activated Delayed Fluorescence from an Excited State Intramolecular Proton Transfer System”
J. Phys. Chem. Lett. 2020, 11, 3305–3312.
[42] Yuya Oyama, Masashi Mamada*, Atul Shukla, Evan G. Moore, Shih-Chun Lo, Ebinazar B. Namdas and Chihaya Adachi*
“Design strategy for robust organic semiconductor laser dyes”
ACS. Mater. Lett. 2020, 2, 161–167.
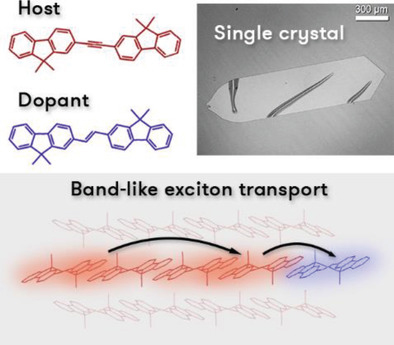
[41] Paulius Baronas*, Gediminas Kreiza, Masashi Mamada, Satoshi Maedera, Povilas Adomėnas, Ona Adomėnienė, Karolis Kazlauskas, Chihaya Adachi, Saulius Juršėnas*
“Enhanced energy transfer in doped bifluorene single crystals: prospects for organic lasers”
Adv. Opt. Mater. 2020, 8, 1901670.
[40] Tanguy Jousselin-Oba, Masashi Mamada*, Jérôme Marrot, Antoine Maignan, Chihaya Adachi, Abderrahim Yassar, and Michel Frigoli*
“Excellent Semiconductors Based on Tetracenotetracene and Pentacenopentacene: From Stable Closed-Shell to Singlet Open-Shell”
J. Am. Chem. Soc. 2019, 141, 9373–9381.
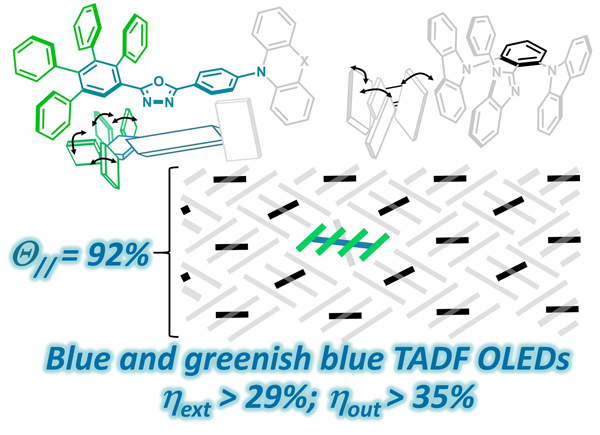
[39] Yi-Ting Lee, Po-Chen Tseng, Takeshi Komino, Masashi Mamada, Ruby Janet Ortiz, Man-kit Leung, Tien-Lung Chiu, Chi-Feng Lin, Jiun-Haw Lee*, Chihaya Adachi*, Chao-Tsen Chen*, Chin-Ti Chen*
“Simple molecular-engineering approach for enhancing orientation and out-coupling efficiency of thermally activated delayed fluorescent emitters without red-shifting emission”
ACS Appl. Mater. Interfaces, 2018, 10, 43842–43849.
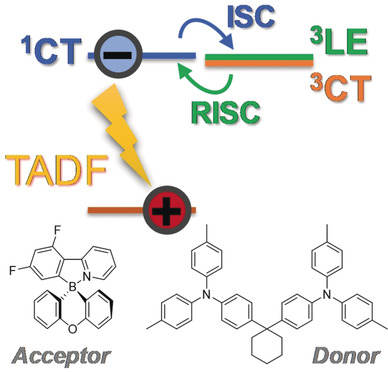
[38] Van T. N. Mai, Atul Shukla, Masashi Mamada, Satoshi Maedera, Paul E. Shaw, Jan Sobus, Ilene Allison, Chihaya Adachi*, Ebinazar B. Namdas*, Shih-Chun Lo*
“Low ASE Threshold and Efficient Electroluminescence from a Carbazole Derivatized Excited State Intramolecular Proton Transfer (ESIPT) Dye”
ACS Photonics, 2018, 5, 4447–4455
Selected as a supplementary cover picture [image]
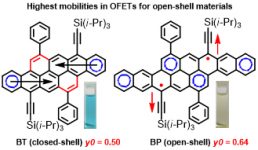
[37] Masashi Mamada*, Guojian Tian, Hajime Nakanotani, Jianhua Su, Chihaya Adachi*
“The Importance of Excited‐State Energy Alignment for Efficient Exciplex Systems Based on a Study of Phenylpyridinato Boron Derivatives”
Angew. Chem. Int. Ed., 2018, 57, 12380–12384
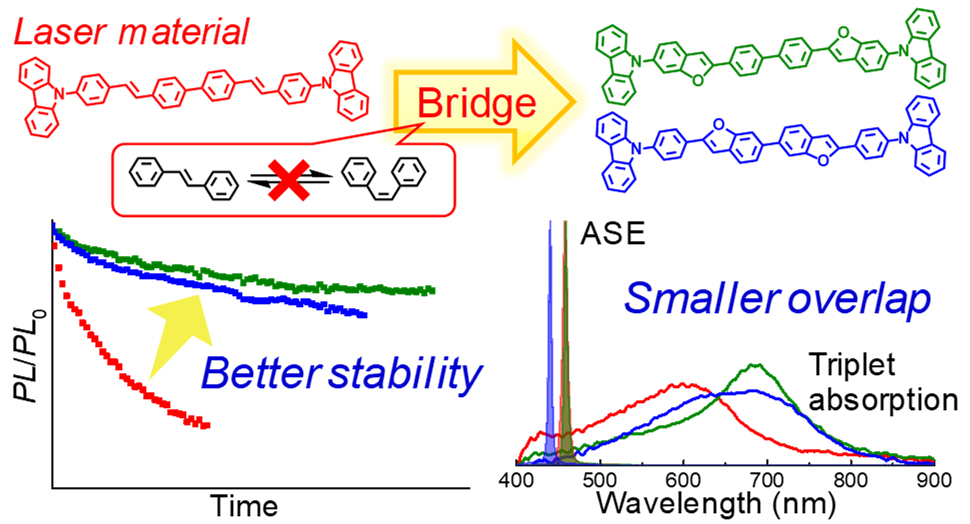
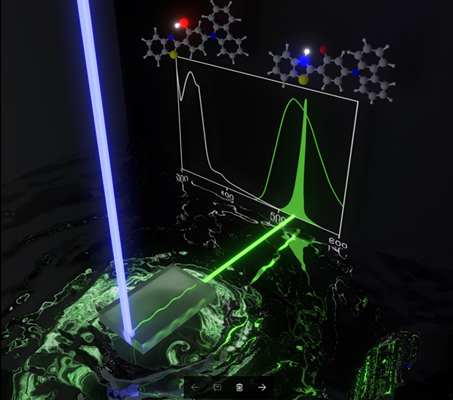
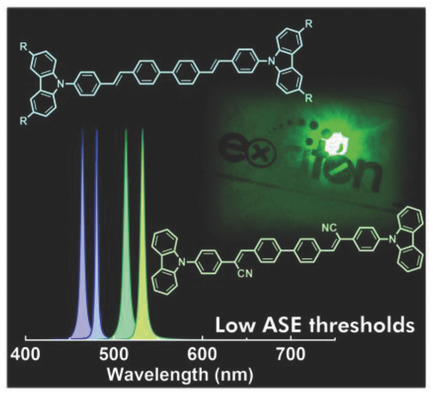
[36] Masashi Mamada*, Toshiya Fukunaga, Fatima Bencheikh, Atula S. D. Sandanayaka, Chihaya Adachi*
“Low Amplified Spontaneous Emission Threshold from Organic Dyes Based on Bis-stilbene”
Adv. Funct. Mater., 2018, 28, 1802130
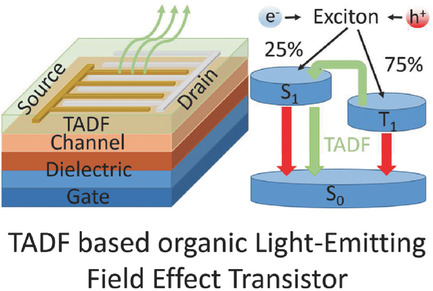
[35] Jan Sobus, Fatima Bencheikh, Masashi Mamada, Robert Wawrzinek, Jean-Charles Ribierre, Chihaya Adachi*, Shih-Chun Lo*, Ebinazar B. Namdas*
“High performance p-and n-type light-emitting field-effect transistors employing thermally activated delayed fluorescence”
Adv. Funct. Mater., 2018, 28, 1800340
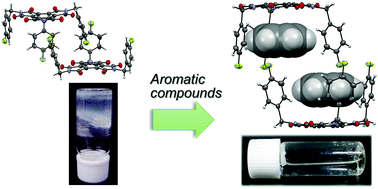
[34] Masashi Mamada*, Tsuyoshi Minami*, Hiroshi Katagiri, Takafumi Omiya, Shizuo Tokito
“One-step, green synthesis of a supramolecular organogelator based on mellitic triimide for the recognition of aromatic compounds”
Chem. Commun., 2017, 53, 8834–8837
Selected as a back cover picture [image]
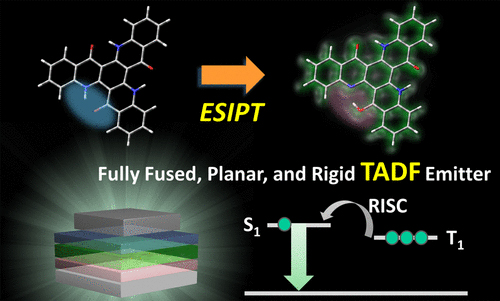
[33] Masashi Mamada*, Ko Inada, Takeshi Komino, William J. Potscavage, Jr., Hajime Nakanotani, Chihaya Adachi*
“Highly Efficient Thermally Activated Delayed Fluorescence from an Excited-State Intramolecular Proton Transfer System”
ACS Cent. Sci., 2017, 3, 769–777
[32] Yuta Ogawa, Kazuhiro Yamamoto, Chiyo Miura, Shigeki Tamura, Mitsuki Saito, Masashi Mamada, Daisuke Kumaki, Shizuo Tokito, Hiroshi Katagiri*
“Asymmetric Alkylthienyl Thienoacenes Derived from Anthra[2,3-b]thieno[2,3-d]thiophene for Solution-Processable Organic Semiconductors”
ACS Appl. Mater. Interfaces 2017, 9, 9902–9909
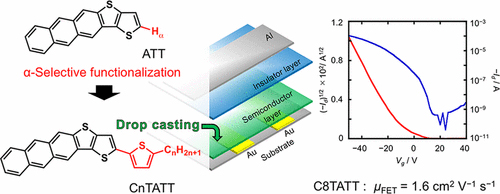
[31] Kamal Sbargoud, Masashi Mamada*, Tanguy Jousselin-Oba, Yasunori Takeda, Shizuo Tokito, Abderrahim Yassar, Jérôme Marrot, Michel Frigoli*
“Low-Band Gap Bistetracene-Based Organic Semiconductors Exhibiting Air Stability, High Aromaticity and Mobility”
Chem. Eur. J. 2017, 23, 5076–5080
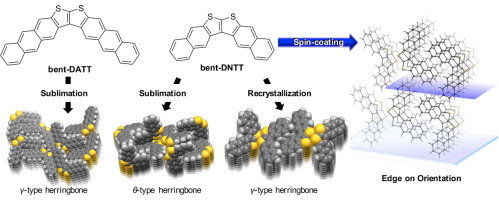
[30] Yuta Ogawa, Erika Takiguchi, Masashi Mamada, Daisuke Kumaki, Shizuo Tokito, Hiroshi Katagiri*
“Synthesis, crystal structure, and FET characteristics of thieno[2,3-b]thiophene-based bent-thienoacenes”
“Synthesis, crystal structure, and FET characteristics of thieno[2,3-b]thiophene-based bent-thienoacenes”
Tetrahedron Lett. 2017, 58, 963–967
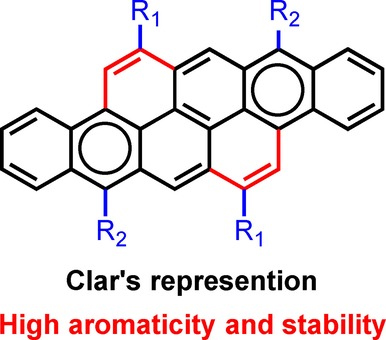
[29] Masashi Mamada*, Hiroshi Katagiri, Tomo Sakanoue, Shizuo Tokito
“Crystal Structure and Theoretical Investigation of Charge Transport Properties of Fullerene Derivatives”
Chem. Lett. 2016, 45,1421–1424
Editor’s Choice article
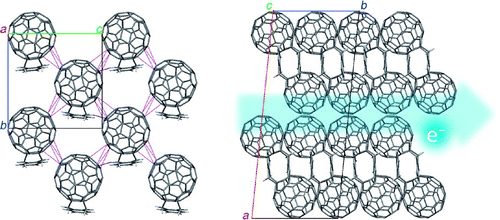
[28] Masataka Ikeshima, Masashi Mamada, Tsuyoshi Minami, Shizuo Tokito, Shuji Okada*
“Synthesis and solid-state polymerization of diacetylene derivatives directly substituted with a phenylcarbazole moiety”
Polym. J. 2016, 48, 1013–1018
Selected as a cover picture

[27] Yasunori Takeda, Kazuma Hayasaka, Rei Shiwaku, Koji Yokosawa, Takeo Shiba, Masashi Mamada, Daisuke Kumaki, Kenjiro Fukuda, Shizuo Tokito*
“Fabrication of Ultra-Thin Printed Organic TFT CMOS Logic Circuits Optimized for Low-Voltage Wearable Sensor Applications”
Sci. Rep. 2016, 6, 25714
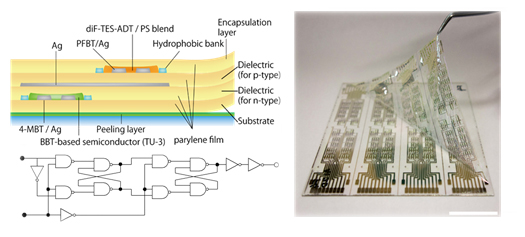
[26] Masashi Mamada*, Harunori Fujita, Kazuaki Kakita, Hidetaka Shima, Yasuhiro Yoneda, Yasuhiro Tanaka*, Shizuo Tokito
“Crystal structure and modeled charge carrier mobility of benzobis(thiadiazole) derivatives”
New J. Chem. 2016, 40, 1403–1411.
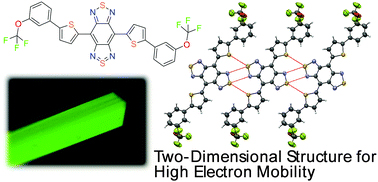
[25] Makoto Mizukami*, Shinya Oku, Seung-Il Cho, Masahiro Tatetsu, Miho Abiko, Masashi Mamada, Tomo Sakanoue, Yoshiyuki Suzuri, Junji Kido, Shizuo Tokito*
“A Solution-Processed Organic Thin-Film Transistor Backplane for Flexible Multiphoton Emission Organic Light-Emitting Diode Displays”
IEEE Electron Device Lett. 2015, 36, 841–843.

[24] Kamal Sbargoud, Masashi Mamada*, Jérôme Marrot, Shizuo Tokito, Abderrahim Yassar*, Michel Frigoli*
“Diindeno[1,2-b:2′,1′-n]Perylene: a Closed Shell related Chichibabin’s Hydrocarbon, Synthesis, Molecular Packing, Electronic and Charge Transport Properties”
Chem. Sci. 2015, 6, 3402–3409.
DOI: 10.1039/C5SC00652J
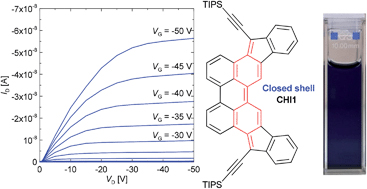
[23] Masataka Ikeshima, Masashi Mamada, Hiroshi Katagiri, Tsuyoshi Minami, Shuji Okada, Shizuo Tokito*
“Synthesis and Solid-State Polymerization of Diacetylene Derivatives with an N-Carbazolylphenyl Group”
Bull. Chem. Soc. Jpn. 2015, 88, 843–849.
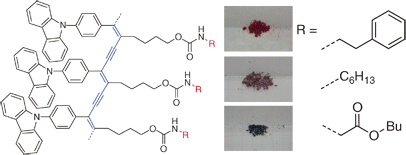
[22] Masashi Mamada, Hidetaka Shima, Yasuhiro Yoneda, Tetsuro Shimano, Natsuko Yamada, Kazuaki Kakita, Toshikazu Machida, Yasuhiro Tanaka*, Sei Aotsuka, Daisuke Kumaki, Shizuo Tokito*
“A Unique Solution Processable n-Type Semiconductor Material Design for High Performance Organic Field-Effect Transistors”
Chem. Mater. 2015, 27, 141–147.
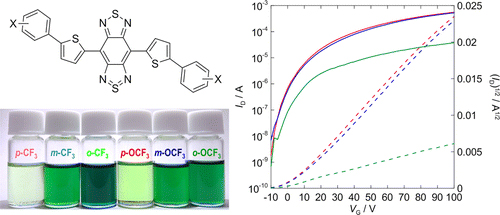
[21] Masashi Mamada*, Hiroshi Katagiri, Tomo Sakanoue, Shizuo Tokito
“Characterization of New Rubrene Analogues with Heteroaryl-Substituents”
Cryst. Growth Des. 2015, 15, 442–448.
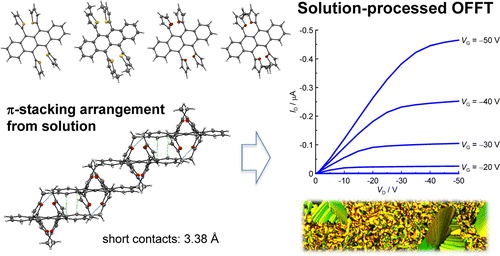
[20] Masashi Mamada, César Perez-Bolivar, Daisuke Kumaki, Nina A. Esipenko, Shizuo Tokito, Pavel Anzenbacher, Jr.*
“Benzimidazole Derivatives: Synthesis, Physical Properties, and n-Type Semiconducting Properties”
Chem. Eur. J. 2014, 20, 11835–11846.
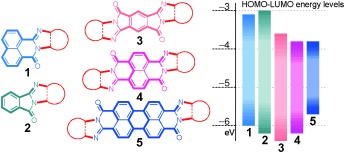
[19] Masashi Mamada*, Taisuke Uemura, Ryo Teraoka, Daisuke Kumaki, Shizuo Tokito
“Synthesis of Narrow Bandgap Polymers based on Benzobis(thiadiazole) and their Application to Organic Transistor Devices”
J. Photopolym. Sci. 2014, 27, 321–326.
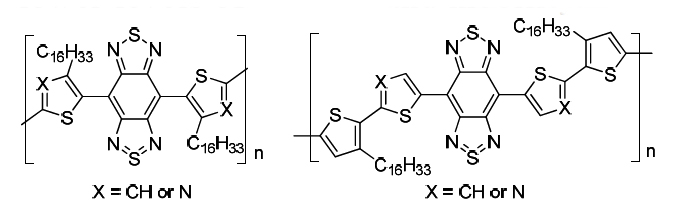
[18] Taisuke Uemura, Masashi Mamada*, Ryo Teraoka, Daisuke Kumaki, Shizuo Tokito*
“Synthesis and Thin-Film Transistor Characterization of Narrow-Gap N-type Semiconducting Polymers based on Benzobis(thiadiazole)”
Chem. Lett. 2014, 43, 402–404.
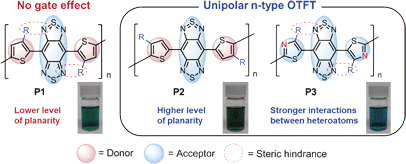
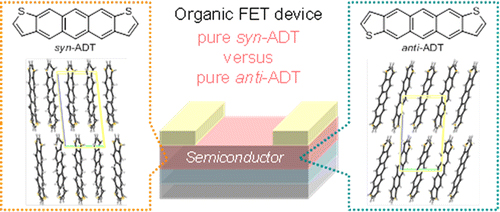
[17] Masashi Mamada*, Hiroshi Katagiri, Makoto Mizukami, Kota Honda, Tsukuru Minamiki, Ryo Teraoka, Taisuke Uemura, Shizuo Tokito
“syn-/anti-Anthradithiophene Derivative Isomer Effects on Semiconducting Properties”
ACS Appl. Mater. Interfaces 2013, 5, 9670–9677.
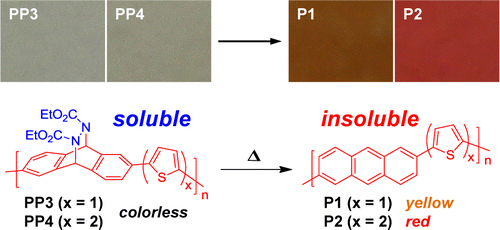
[16] Taisuke Uemura, Masashi Mamada, Daisuke Kumaki, Shizuo Tokito*
“Synthesis of Semiconducting Polymers through Soluble Precursor Polymers with Thermally Removable Groups and Their Application to Organic Transistors”
ACS Macro Lett. 2013, 2, 830–833.
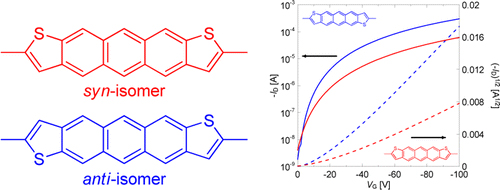
[15] Masashi Mamada*, Tsukuru Minamiki, Hiroshi Katagiri, Shizuo Tokito*
“Synthesis, Physical Properties, and Field-Effect Mobility of Isomerically Pure syn-/anti-Anthradithiophene Derivatives”
Org. Lett. 2012, 14, 4062–4065.
[14] Masashi Mamada, César Pérez-Bolívar, Pavel Anzenbacher, Jr.*
“Green Synthesis of Polycyclic Benzimidazole Derivatives and Organic Semiconductors”
Org. Lett. 2011, 13, 4882–4885.
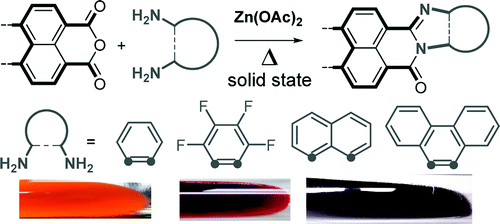
[13] Masashi Mamada, Selin Ergun, César Pérez-Bolívar, Pavel Anzenbacher, Jr.
“Charge transport, carrier balance, and blue electrophosphorescence in diphenyl[4-(triphenylsilyl)phenyl]-phosphine oxide devices.”
Appl. Phys. Lett. 2011, 98, 073305.
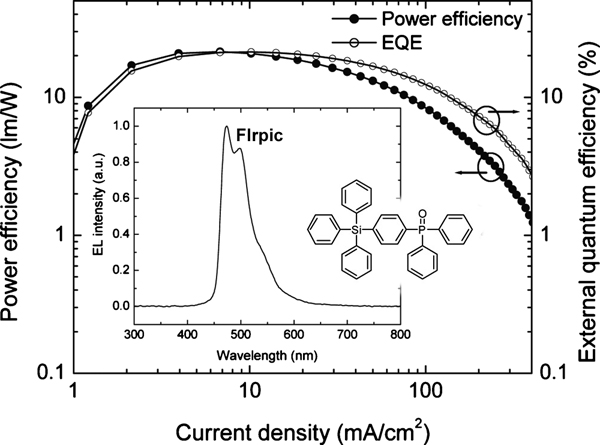
[12] Masashi Mamada, Daisuke Kumaki, Jun-ichi Nishida, Shizuo Tokito, Yoshiro Yamashita*
“Novel Semiconducting Quinone for Air-Stable n-Type Organic Field-Effect Transistors.”
ACS Appl. Mater. Interfaces 2010, 2, 1303–1307.
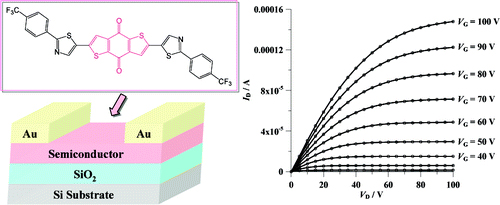
[11] Keiko Omata, Masashi Mamada, Jun-ichi Nishida, Shizuo Tokito, Yoshiro Yamashita
“Organic Field-Effect Transistors Based on π-Extended Dibenzotetrathiafulvalene Analogues with Thiophene Spacers.”
Bull. Chem. Soc. Jpn. 2010, 83, 575–581.
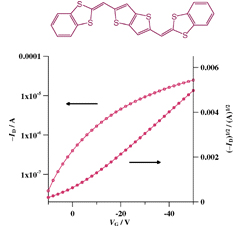
[10] Yoshihide Fujisaki*, Masashi Mamada, Daisuke Kumaki, Shizuo Tokito, Yoshiro Yamashita
“Low-Voltage and Hysteresis-Free N-Type Organic Thin Film Transistor and Complementary Inverter with Bilayer Gate Insulator.”
Jpn. J. Appl. Phys. 2009, 48, 111504.
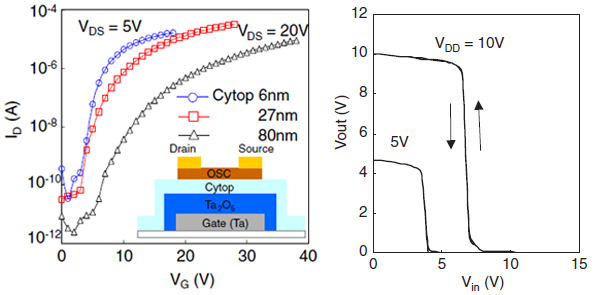
[9] Altanbolag, Masashi Mamada, Jun-ichi Nishida, Yoshiro Yamashita*
“Field-Effect Transistors Based on Tetraphenyl-dipyranylidenes and the Sulfur Analogues.”
Chem. Mater. 2009, 21, 4350–4352.
[8] Masashi Mamada, Yoshiro Yamashita*
“Triclinic Polymorph of Dibenzotetrathiafulvalene.”
Acta Crystallogr. Sect. E 2009, 65, o2083.
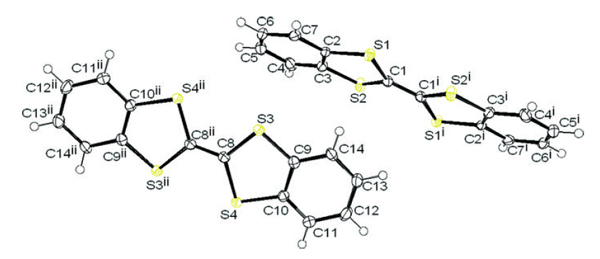
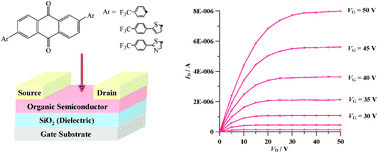
[7] Masashi Mamada, Jun-ichi Nishida, Shiuo Tokito, Yoshiro Yamashita*
“Anthraquinone Derivatives Affording n-Type Organic Thin Film Transistors.”
Chem. Commun. 2009, 16, 2177–2179.
DOI:10.1039/B820520E
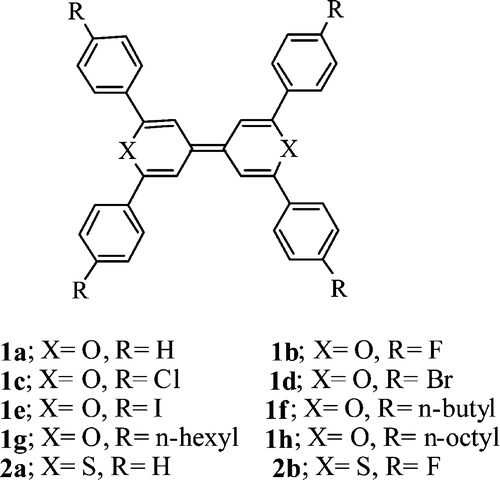
[6] Masashi Mamada, Jun-ichi Nishida, Shizuo Tokito, Yoshiro Yamashita*
“Preparation, Characterization, and Field-effect Transistor Performance of Benzo[1,2-d:4,5-d’]bisthiazole Derivatives.”
Chem. Lett. 2008, 37, 766–767.
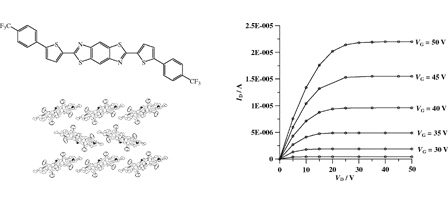
[5] Masashi Mamada, Jun-ichi Nishida, Daisuke Kumaki, Shizuo Tokito, Yoshiro Yamashita*
“High Performance Organic Field-Effect Transistors Based on [2,2′]Bi[naphtho[2,3-b]thiophenyl] with a Simple Structure.”
J. Mater. Chem. 2008, 18, 3442–3447.
DOI:10.1039/B801425F
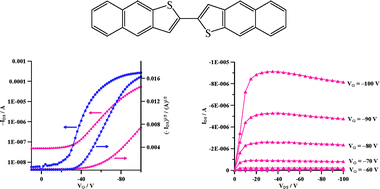
[4] Masashi Mamada, Jun-ichi Nishida, Daisuke Kumaki, Shizuo Tokito, Yoshiro Yamashita*
“n-Type Organic Field-Effect Transistors with High Electron Mobilities Based on Thiazole-thiazolothiazole Conjugated Molecules.”
Chem. Mater. 2007, 19, 5404–5409.
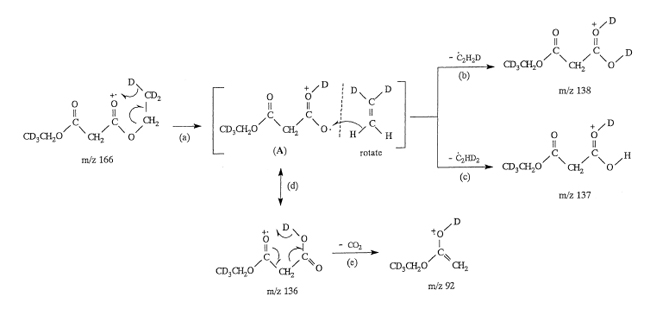
[3] Susumu Tajima*, Daisuke Ishiguro, Masashi Mamada, Satoshi Nakajima
“Unimolecular Reactions of Diethtyl Malonate Cation in Gas-phase.”
J. Mass Spectrom. Soc. Jpn. 2004, 52, 263–270.
[2] Susumu Tajima*, Masashi Mamada, Satoshi Nakajima, Yutaka Takahashi, Nico M. M. Nibbering
“Unimolecular Gas-Phase Reactions of Diethyl Phthalate, Isophthalate, and Terephthalate upon Electron Ionization.”
Aust. J. Chem. 2003, 56, 473–479.
DOI:10.1071/CH03077
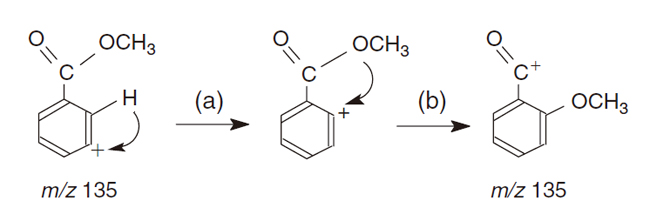
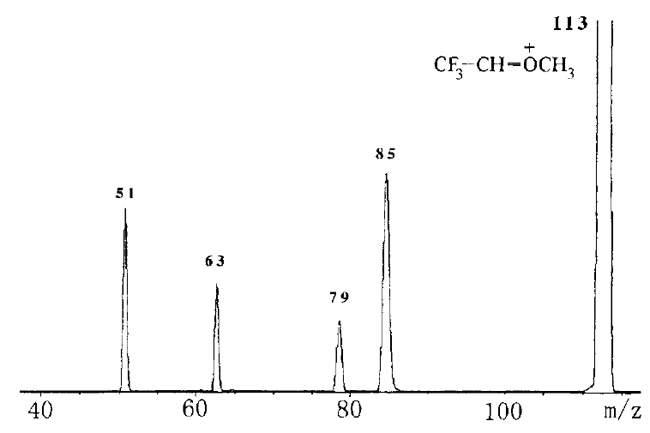
[1] Susumu Tajima*, Shoko Kojima, Yuko Hiroi, Masashi Mamada, Satoshi Nakajima, Nico M. M. Nibbering
“Unimolecular Metastable Decompositions of 1,1,1-Trifluoroisopropyl Methyl Ether upon Electron Ionization.”
Rapid Commun. Mass Spectrom. 2003, 17, 503–506.
DOI:10.1002/rcm.892
著書・解説・総説等
- [3]
- 有機半導体レーザー分子の耐久特性の向上
電気学会誌, 2021, 141, 283–285.
Masashi Mamada
DOI:10.1541/ieejjournal.141.283
- [2]
- 有機半導体レーザー分子の耐久特性の向上
Vacuum and Surface Science, 2021, 64, 4–9.
Chihaya Adachi, Masashi Mamada, Toshinori Matsushima
DOI:10.1380/vss.64.4
- [1]
- Polycyclic Arenes and Heteroarenes: Synthesis, Properties, and Applications
Chapter 11, pp. 277–308. Masashi Mamada and Yoshiro Yamashita, (2015) Wiley
DOI:10.1002/9783527689545
特許
- [8]
- 儘田正史、中野谷一、安達千波矢、ヤンユソク
「有機発光素子、それに用いられる発光材料および遅延蛍光体」
PCT/JP2017/045788 - [7]
- 時任静士、熊木大介、 儘田正史、福田憲二郎、田中康裕、島秀好、米田康洋、垣田一成、小俣洋治、山田奈津子、本間貴志、町田利一、藤田陽師
「ベンゾビス(チアジアゾール)誘導体、およびそれを用いた有機エレクトロニクスデバイス」
特願:PCT/JP2014/072839 - [6]
- 時任静士、熊木大介、 儘田正史、田中康裕、島秀好、米田康洋、垣田一成、小俣洋治、本間貴志、町田利一
「ベンゾビス(チアジアゾール)誘導体、およびそれを用いた有機エレクトロニクスデバイス」
特願:2014-164975 - [5]
- 時任静士、熊木大介、 儘田正史、田中康裕、島秀好、米田康洋、垣田一成、小俣洋治、町田利一
「ベンゾビス(チアジアゾール)誘導体、およびそれを用いた有機エレクトロニクスデバイス」
特願:2014-174786 - [4]
- 儘田正史、時任静士、片桐洋史、坂上知
「テトラセン誘導体及びその合成方法並びにそれを用いた有機エレクトロニクスデバイス」
特願:2014-077476 - [3]
- 時任静士、熊木大介、 儘田正史、田中康裕、島秀好、米田康洋、藤田陽師、垣田一成、小俣洋治、山田奈津子、本間貴志、町田利一
「ベンゾビス(チアジアゾール)誘導体、それを含むインク、およびそれを用いた有機エレクトロニクスデバイス」
特願:2014-026969 - [2]
- 儘田正史、時任静士、片桐洋史、熊木大介、渡辺翔一
「テトラアミン誘導体及びその合成方法並びにそれを用いた有機エレクトロニクスデバイス」
特願:2014-027138 - [1]
- 時任静士、熊木大介、 儘田正史、田中康裕、島秀好、米田康洋、垣田一成、小俣洋治、山田奈津子、本間貴志、町田利一
「ベンゾビス(チアジアゾール)誘導体、およびそれを用いた有機エレクトロニクスデバイス」
特願:2013-196221
国際会議発表
- [6]
- (Invited) Synthesis, Aromaticity and Application to OFET and OLED of Peri-Pentacenopentacene
M. Mamada, Tanguy Jousselin-Oba, Karen Wright, Jérome Marrot, Chihaya Adachi, Abderrahim Yassar, Michel Frigoli
231st ECS Meeting, 2022, Vancouver. - [5]
- (Invited) Development of highly emissive laser dyes for organic semiconductor laser diodes
M. Mamada, Chihaya Adachi
The 13th Asian Conference on Organic Electronics (A-COE) 2021, Online. - [4]
- (Invited) Singlet Biradical Compounds: Development and Application to Organic Electronic Devices
M. Mamada, Tanguy Jousselin-Oba, Chihaya Adachi, Michel Frigoli
Asian International Symposium – Organic Crystals, Chiba, Japan. - [3]
- (Invited) A novel molecular design strategy for thermally activated delayed fluorescence materials
M. Mamada
France-Japan Workshop on Optoelectronics & Photonics 2018, Paris, France. - [2]
- (Invited) High efficiency thermally activated delayed fluorescence through an excited-state intramolecular proton transfer
M. Mamada, K. Inada, T. Komino, W. J. Potscavage, H. Nakanotani, C. Adachi
SPIE Organic Light Emitting Materials and Devices XXI 2017, San Diego, USA. - [1]
- (Invited) Isomer Effects on Semiconducting Properties in organic transistors
M. Mamada, S. Tokito
The 5th Asian Conference on Organic Electronics (A-COE) 2013, POSTECH, Pohang, Korea.