Publication
(2020)
(2019)
(2018)
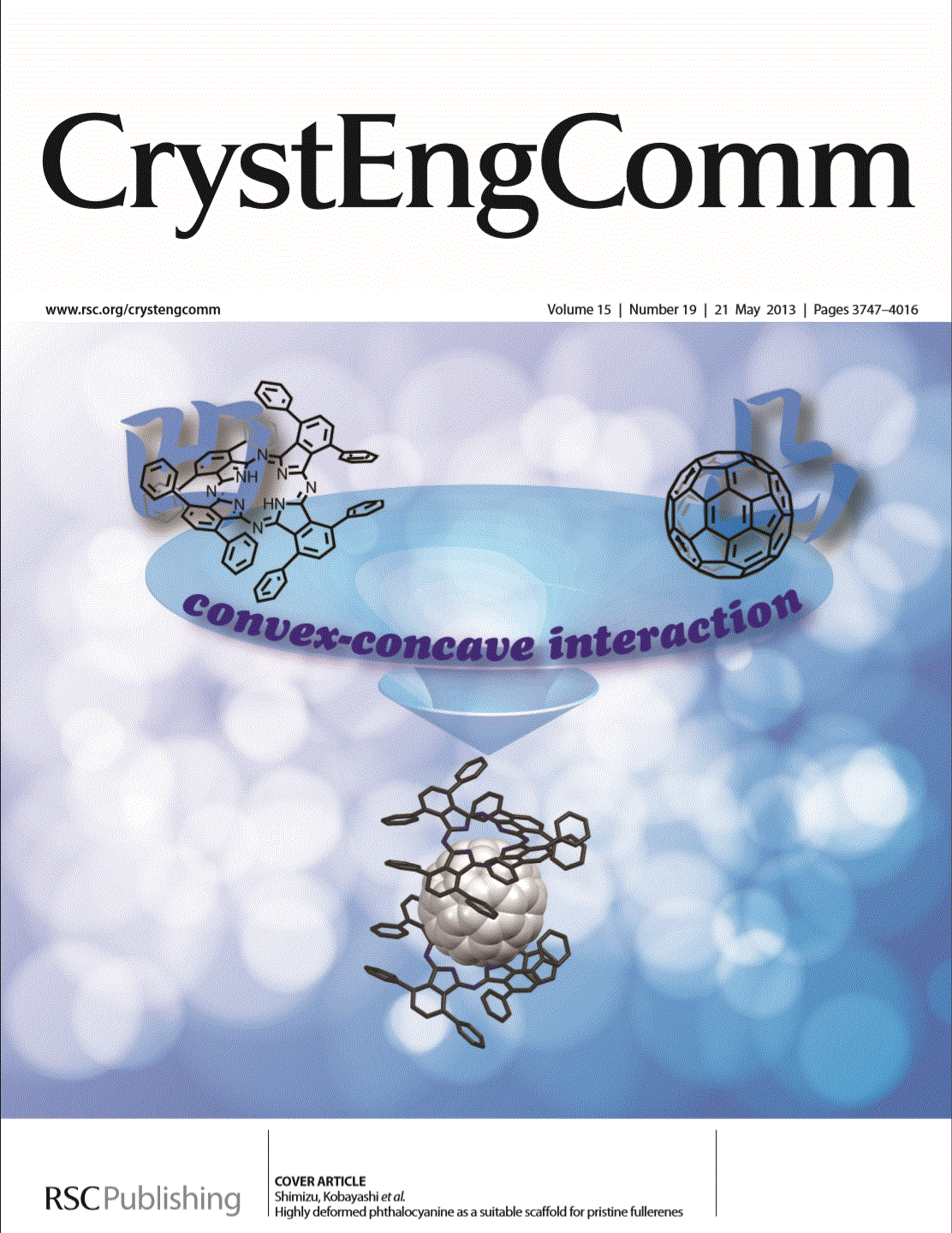
5,15-Diheteroporphyrins Synthesized from α,α'-Dihalodipyrrin as a Key Building Block
Shimizu, S.
HETEROCYCLES 2020, 100, 1123-1162. [Link]
Shimizu, S.
HETEROCYCLES 2020, 100, 1123-1162. [Link]

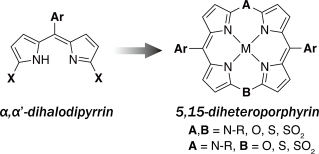
TTF-Annulated Silicon Phthalocyanine Oligomers and Their External-Stimuli-Responsive Orientational Ordering
Shiina, Y.; Kage, Y.; Furukawa, K.; Wang, H.; Yoshikawa, H.; Furuta, H.; Kobayashi, N.; Shimizu, S.
Angew. Chem. Int. Ed. in press. [Link]
Shiina, Y.; Kage, Y.; Furukawa, K.; Wang, H.; Yoshikawa, H.; Furuta, H.; Kobayashi, N.; Shimizu, S.
Angew. Chem. Int. Ed. in press. [Link]

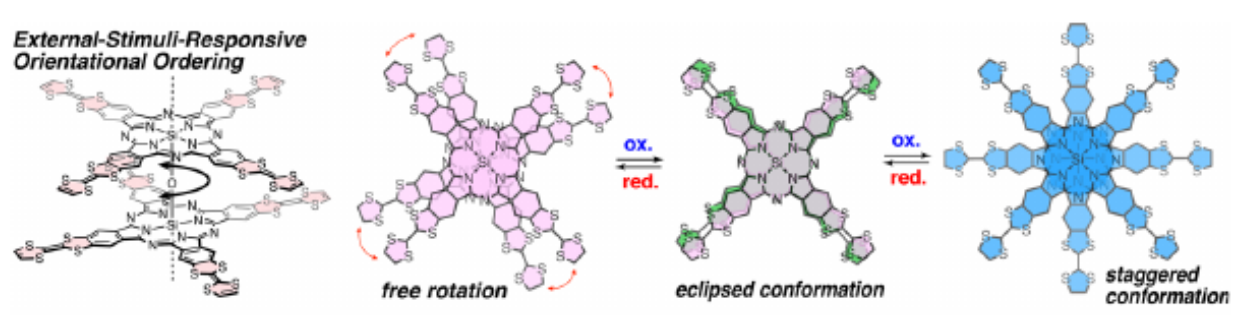
Near-Infrared Absorbing Pyrrolopyrrole Aza-BODIPY-Based Donor-Acceptor Polymers with Reasonable Photoresponse
Feng, R.; Sato, N.; Nomura, M.; Saeki, A.; Nakanotani, H.; Adachi, C.; Yasuda, T.; Furuta, H.; Shimizu, S.
J. Mater. Chem. C 2020, 8, 8770-8776. [Link]
Feng, R.; Sato, N.; Nomura, M.; Saeki, A.; Nakanotani, H.; Adachi, C.; Yasuda, T.; Furuta, H.; Shimizu, S.
J. Mater. Chem. C 2020, 8, 8770-8776. [Link]

Copper 1,19-Diaza-21,24-dicarbacorrole: A Corrole Analogue with an N-N Linkage Stabilizes a Ground-State Singlet Organocopper Species
Basumatary, B.; Hashiguchi, I.; Mori, S.; Shimizu, S.; Ishida, M.; Furuta, H.
Angew. Chem. Int. Ed. 2020, 59, 15897-15901. [Link]
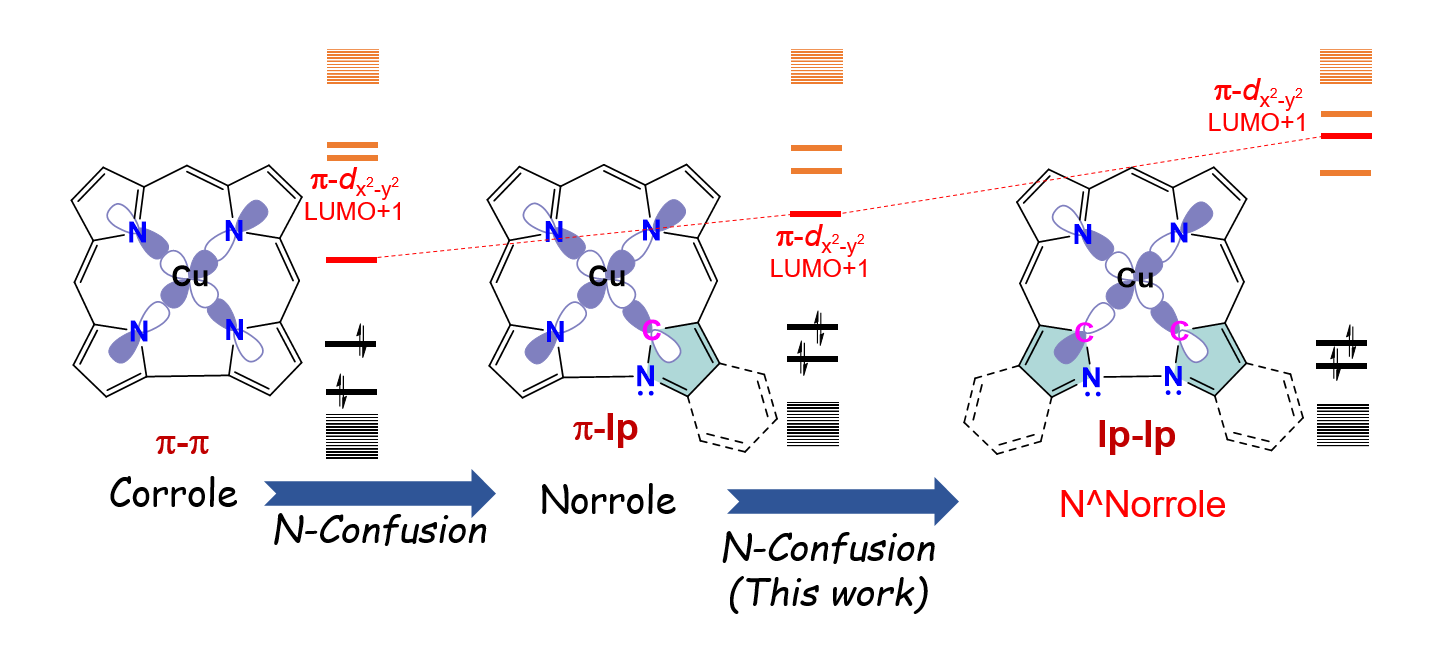
Basumatary, B.; Hashiguchi, I.; Mori, S.; Shimizu, S.; Ishida, M.; Furuta, H.
Angew. Chem. Int. Ed. 2020, 59, 15897-15901. [Link]

Rational Design of Pyrrolopyrrole-Aza-BODIPY-Based Acceptor-Donor-Acceptor Triad for Organic Photovoltaics Application
Feng, R.; Sato, N.; Yasuda, T.; Furuta, H.; Shimizu, S.
Chem. Commun. 2020, 56, 2975-2978. [Link]
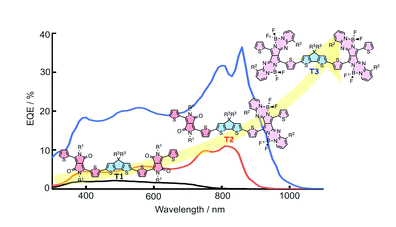
Feng, R.; Sato, N.; Yasuda, T.; Furuta, H.; Shimizu, S.
Chem. Commun. 2020, 56, 2975-2978. [Link]

Tungsten(VI) Complex of N-Fused Porphyrin Absorbing Near-Infrared Light beyond 1000 nm
Yamamoto, T.; Abraham, J. A.; Mori, S.; Toganoh, M.; Shimizu, S.; Ishida, M.; Furuta, H.
Chem. Asian. J. 2020, 15, 748-752. [Link]
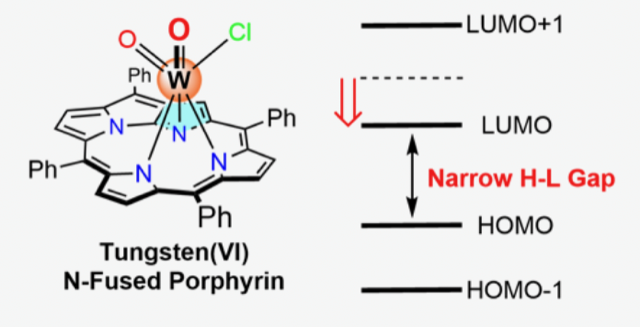
Yamamoto, T.; Abraham, J. A.; Mori, S.; Toganoh, M.; Shimizu, S.; Ishida, M.; Furuta, H.
Chem. Asian. J. 2020, 15, 748-752. [Link]

Subphthalocyanine-Stoppered [2]Rotaxanes: Synthesis and Size/Energy Threshold of Slippage
Kage, Y.; Shimizu, S; Kociok-Köhn, G.; Furuta, H.; Pantoş, G. D.
Org. Lett. 2020, 22, 1096-1101. [Link]
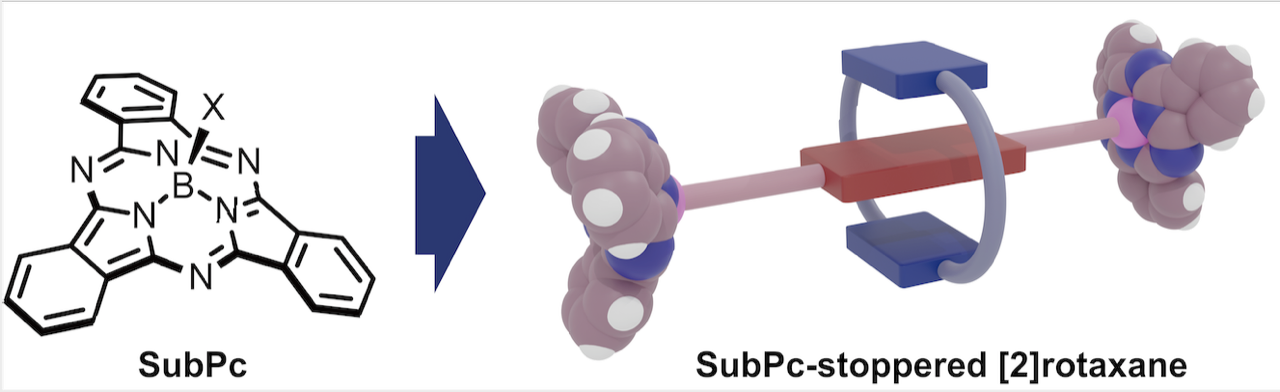
Kage, Y.; Shimizu, S; Kociok-Köhn, G.; Furuta, H.; Pantoş, G. D.
Org. Lett. 2020, 22, 1096-1101. [Link]

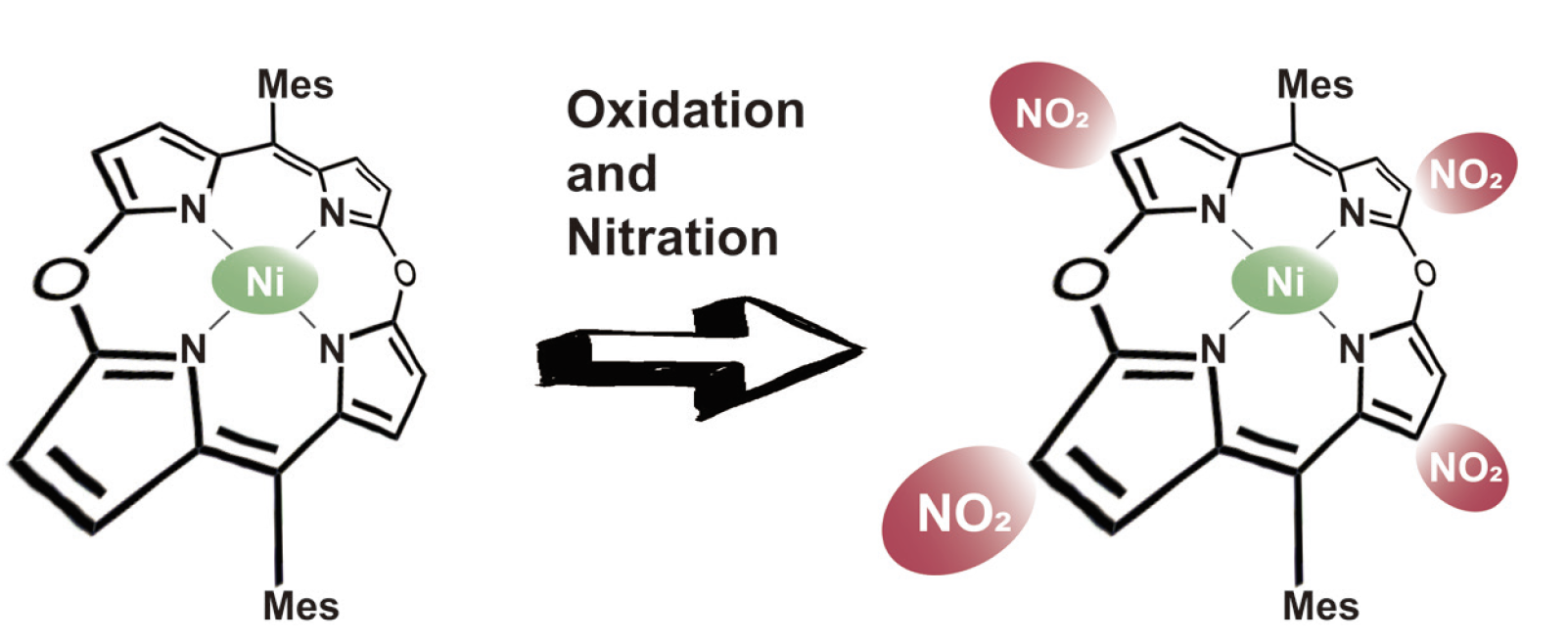
Oxidative Nitration Reaction of Antiaromatic 5,15-Dioxaporphyrin
Nishiyama, A.; Tanaka, Y.; Mori, S.; Furuta, H.; Shimizu, S.
J. Porphyrins Phthalocyanines 2020, 24, 355-361. [Link]
Nishiyama, A.; Tanaka, Y.; Mori, S.; Furuta, H.; Shimizu, S.
J. Porphyrins Phthalocyanines 2020, 24, 355-361. [Link]

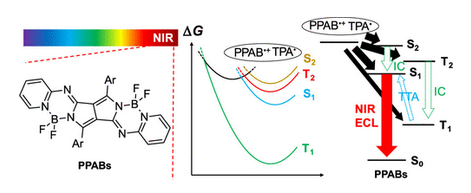
Efficient Electrogenerated Chemiluminescence of Pyrrolopyrrole Aza-BODIPYs in the Near-Infrared Region with Tripropylamine: Involving Formation of S2 and T2 States
Ishimatsu, R.; Shintaku, H.; Kage, Y.; Kamioka, M.; Shimizu, S.; Nakano, K.; Furuta, H.; Imato, T.
J. Am. Chem. Soc. 2019, 141, 11791-11795. [Link]
Ishimatsu, R.; Shintaku, H.; Kage, Y.; Kamioka, M.; Shimizu, S.; Nakano, K.; Furuta, H.; Imato, T.
J. Am. Chem. Soc. 2019, 141, 11791-11795. [Link]

aza-BODIPY synthesis towards vis/NIR functional chromophores based on a Schiff base forming reaction protocol using lactams and heteroaromatic amines
Shimizu, S.
Chem. Commun. 2019, 55, 8722-8743. [Link]
Shimizu, S.
Chem. Commun. 2019, 55, 8722-8743. [Link]
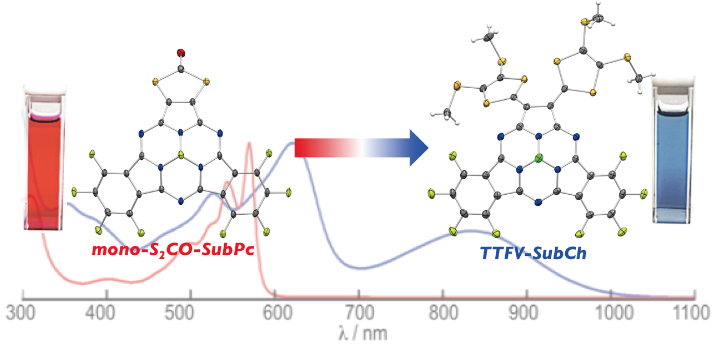
Bis(1,3-dithiol-2-ylidene)-Substituted Subtriazachlorin: A Subphthalocyanine Analogue with Redox Properties
Wang, Y.; Mori, S.; Furuta, H.; Shimizu, S.
Angew. Chem. Int. Ed. 2019, 58, 10975-10979. [Link]
Wang, Y.; Mori, S.; Furuta, H.; Shimizu, S.
Angew. Chem. Int. Ed. 2019, 58, 10975-10979. [Link]

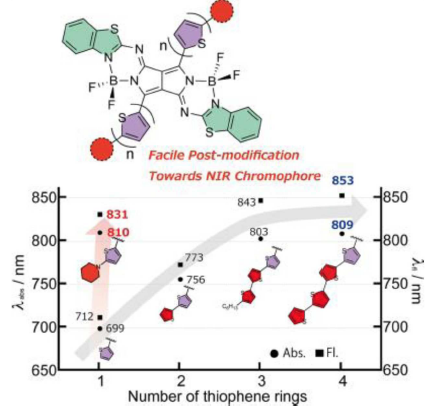
Pyrrolopyrrole Aza-BODIPY Analogues as Near-Infrared Chromophore and Fluorophore: Substituent Effects on the Red-shifts of Absorption and Emission
Kage, Y.; Karasaki, H.; Mori, S.; Furuta, H.; Shimizu, S.
ChemPlusChem 2019, 84, 1-6. [Link]
Kage, Y.; Karasaki, H.; Mori, S.; Furuta, H.; Shimizu, S.
ChemPlusChem 2019, 84, 1-6. [Link]

1,3-Dithiole-2-one-fused Subphthalocyanine and Subporphyrazine: Synthesis and Properties Arising from the 1,3-Dithiole-2-one Units
Wang, Y.; Uchihara, K.; Mori, S.; Furuta, H.; Shimizu, S.
Org. Lett. 2019, 21, 3103-3107. [Link]
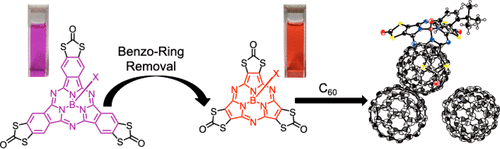
Wang, Y.; Uchihara, K.; Mori, S.; Furuta, H.; Shimizu, S.
Org. Lett. 2019, 21, 3103-3107. [Link]

N-Confused Porphyrin-aza-Dipyrrin Chimera: A Versatile Metal Coordination Ligand Using its Unique NH Tautomerism
Fukuda, M.; Mori, S.; Furuta, H.; Shimizu, S.
Chem. Asian J. 2019, 14, 1697-1702. [Link]
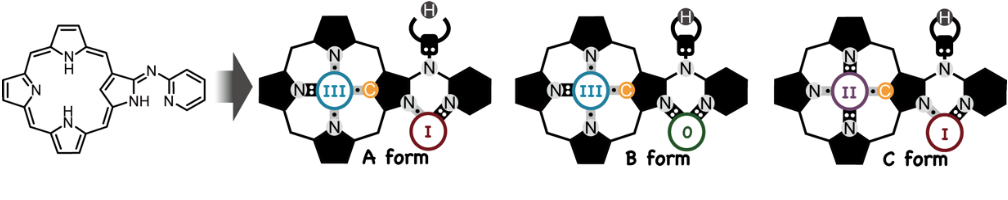
Fukuda, M.; Mori, S.; Furuta, H.; Shimizu, S.
Chem. Asian J. 2019, 14, 1697-1702. [Link]

Synthesis and properties of redox‐switchable zinc complexes of 10,15,20‐triaryl‐15‐aza‐5‐oxaporphyrin
Sudoh, K.; Furukawa, K.; Nakano, H.; Shimizu, S.; Matano, Y.
Heteroatom Chemistry 2018, 29, e21456. [Link]
Sudoh, K.; Furukawa, K.; Nakano, H.; Shimizu, S.; Matano, Y.
Heteroatom Chemistry 2018, 29, e21456. [Link]
Rational Synthesis of Antiaromatic 5,15‐Dioxaporphyrin and Oxidation into β,β‐Linked Dimers
Nishiyama, A.; Fukuda, M.; Mori, S.; Furukawa, K.; Fliegl, H.; Furuta, H.; Shimizu, S.
Angew. Chem. Int. Ed. 2018, 57, 9728-9733. [Link]
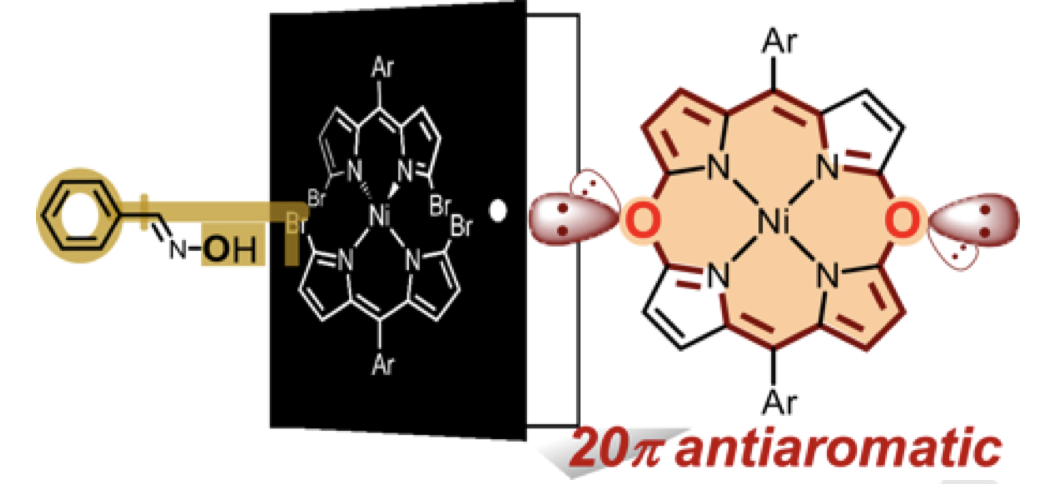
Nishiyama, A.; Fukuda, M.; Mori, S.; Furukawa, K.; Fliegl, H.; Furuta, H.; Shimizu, S.
Angew. Chem. Int. Ed. 2018, 57, 9728-9733. [Link]

The First Silicon(IV) Corrole Complexes: Synthesis, Structures, Properties, and Formation of a µ‐Oxo Dimer
Ueta, K.; Fukuda, M.; Kim, G.; Shimizu, S.; Tanaka, T.; Kim, D.; Osuka, A.
Chem. Eur. J. 2018, 24, 7637-7646. [Link]
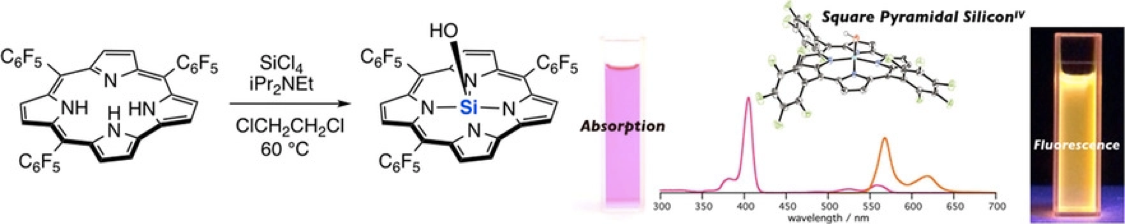
Ueta, K.; Fukuda, M.; Kim, G.; Shimizu, S.; Tanaka, T.; Kim, D.; Osuka, A.
Chem. Eur. J. 2018, 24, 7637-7646. [Link]

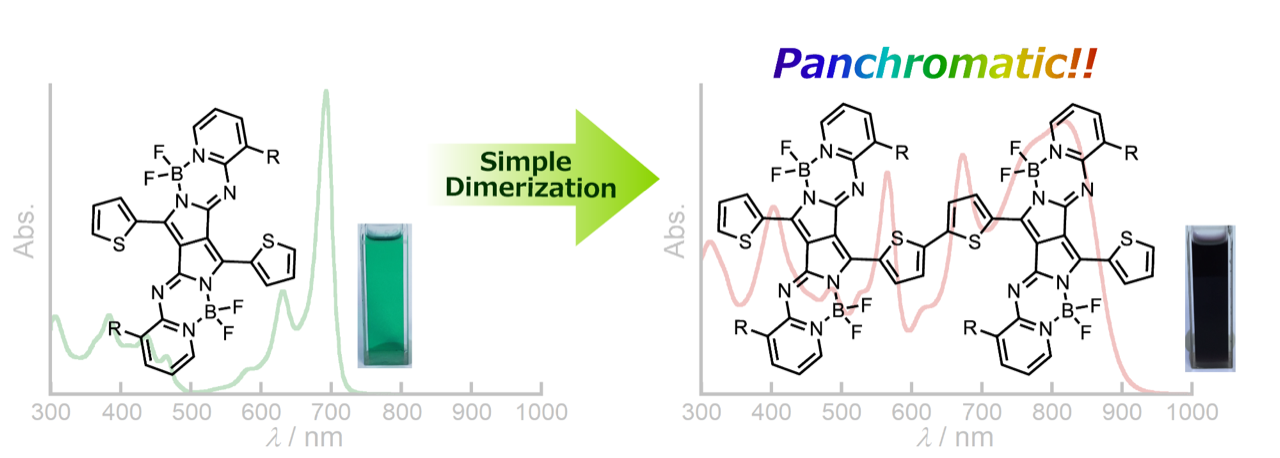
Blackening of Aza-BODIPY Analogues by Simple Dimerization: Panchromatic Absorption of a Pyrrolopyrrole Aza-BODIPY Dimer
Kage, Y.; Mori, S.; Ide, M.; Saeki, A.; Furuta, H.; Shimizu, S.
Mater. Chem. Front. 2018, 2, 112-120. [Link]
Kage, Y.; Mori, S.; Ide, M.; Saeki, A.; Furuta, H.; Shimizu, S.
Mater. Chem. Front. 2018, 2, 112-120. [Link]

(2017)
(2016)
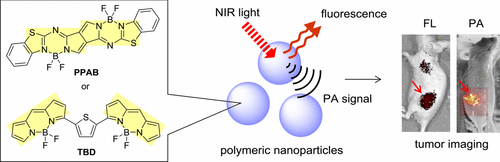
Polymeric Self-Assemblies with Boron-Containing Near-Infrared Dye Dimers for Photoacoustic Imaging Probes
Miki, K.; Enomoto, A.; Inoue, T.; Nabeshima, T.; Saino, S.; Shimizu, S.; Matsuoka, H.; Ohe, K.
Biomacromolecules, 2017, 18, 249–256. [Link]
Miki, K.; Enomoto, A.; Inoue, T.; Nabeshima, T.; Saino, S.; Shimizu, S.; Matsuoka, H.; Ohe, K.
Biomacromolecules, 2017, 18, 249–256. [Link]

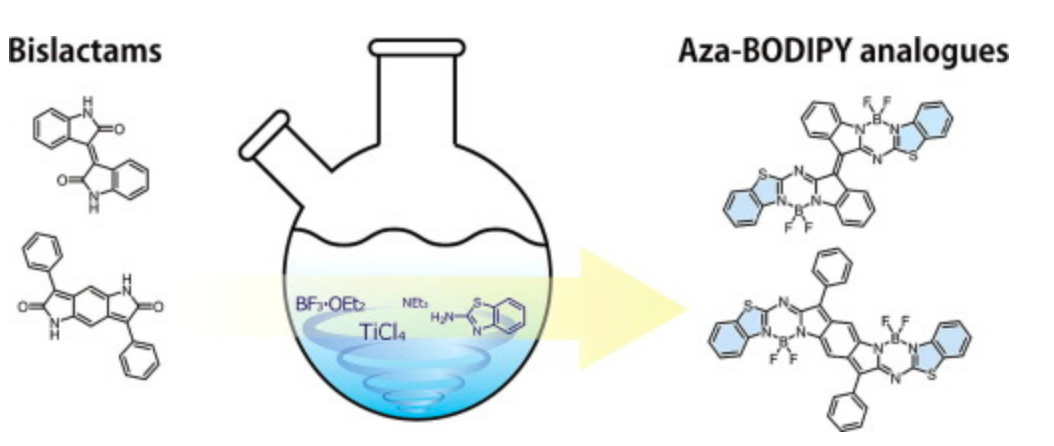
Facile Synthesis of Dimeric Aza-BODIPY Analogues from Electron-Deficient Bislactams and Their Intriguing Optical and Electrochemical Properties
Tamada, M.; Iino, T.; Wang, Y.; Ide, M.; Saeki, A.; Furuta, H., Kobayashi, N.; Shimizu, S.
Tetrahedron Lett. 2017, 58, 3151-3154. [Link]
Tamada, M.; Iino, T.; Wang, Y.; Ide, M.; Saeki, A.; Furuta, H., Kobayashi, N.; Shimizu, S.
Tetrahedron Lett. 2017, 58, 3151-3154. [Link]

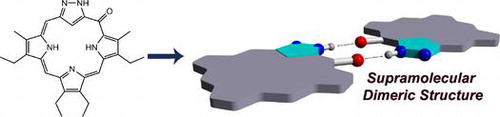
Supramolecular Dimeric Structures of Pyrazole-containing Meso-Oxo Carbaphlorin Analogues
Ishida, M.; Fujimoto, H.; Morimoto, T.; Mori, S.; Toganoh, M.; Shimizu, S.; Furuta, H.
Supramol. Chem. 2017, 29, 8-16 [Link]
Ishida, M.; Fujimoto, H.; Morimoto, T.; Mori, S.; Toganoh, M.; Shimizu, S.; Furuta, H.
Supramol. Chem. 2017, 29, 8-16 [Link]

Phenylene-Bridged Expanded Porphyrazines
Iizuka, F.; Kage, Y.; Kobayashi, N.; Furuta, H.; Shimizu, S.
ChemPlusChem 2017, 82 1021-1024 [Link]
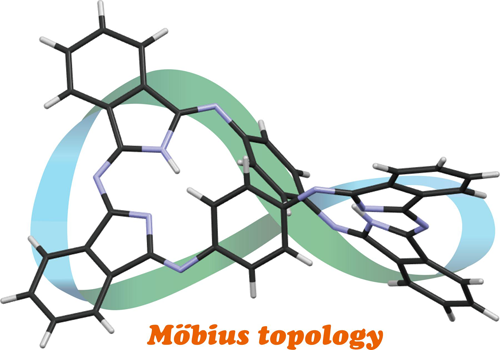
Iizuka, F.; Kage, Y.; Kobayashi, N.; Furuta, H.; Shimizu, S.
ChemPlusChem 2017, 82 1021-1024 [Link]

Recent Advances in Subporphyrins and Triphyrin Analogues: Contracted Porphyrins Comprising Three Pyrrole Rings
Soji Shimizu
Chem. Rev. 2017 117 2730-2784[Link]
Soji Shimizu
Chem. Rev. 2017 117 2730-2784[Link]

Stacked antiaromatic porphyrins
Ryo Nozawa, Hiroko Tanaka, Won-Young Cha, Yongseok Hong, Ichiro Hisaki, Soji Shimizu, Ji-Young Shin, Tim Kowalczyk, Stephan Irle, Dongho Kim & Hiroshi Shinokubo
Nat. Chem., 2016, 30 [Link]
Ryo Nozawa, Hiroko Tanaka, Won-Young Cha, Yongseok Hong, Ichiro Hisaki, Soji Shimizu, Ji-Young Shin, Tim Kowalczyk, Stephan Irle, Dongho Kim & Hiroshi Shinokubo
Nat. Chem., 2016, 30 [Link]
Pyrene-Bridged Boron Subphthalocyanine Dimers: Combination of Planar and Bowl-Shaped π-Conjugated Systems for Creating Uniquely Curved π-Conjugated Systems
Nakano, S.; Kage, Y.; Furuta, H.; Kobayashi, N.; Shimizu, S.
Chem. Eur. J.2016, 22, 7706-7710 [Link]
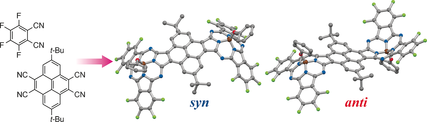
Nakano, S.; Kage, Y.; Furuta, H.; Kobayashi, N.; Shimizu, S.
Chem. Eur. J.2016, 22, 7706-7710 [Link]

A Novel Isoindole-Containing Polyaromatic Hydrocarbon Unexpectedly Formed during the Synthesis of Meso-2,6-Dichlorophenyl-Substituted Tribenzosubporphyrin
Shiina, Y.; Karasaki, H.; Mori, S.; Kobayashi, N.; Furuta, H.; Shimizu, S.
J. Porphyrin Phthalocyanines 2016, 20, 1049 [Link]
Shiina, Y.; Karasaki, H.; Mori, S.; Kobayashi, N.; Furuta, H.; Shimizu, S.
J. Porphyrin Phthalocyanines 2016, 20, 1049 [Link]
Core-Modified Phthalocyanines and Subphthalocyanines: A Synthetic Strategy towards Core-Modification and Novel Properties Arising from the Inner Ring-Expansion
Shimizu, S.; Furuta, H.
Macroheterocycles2015, 54, 7323-7327 [Link]
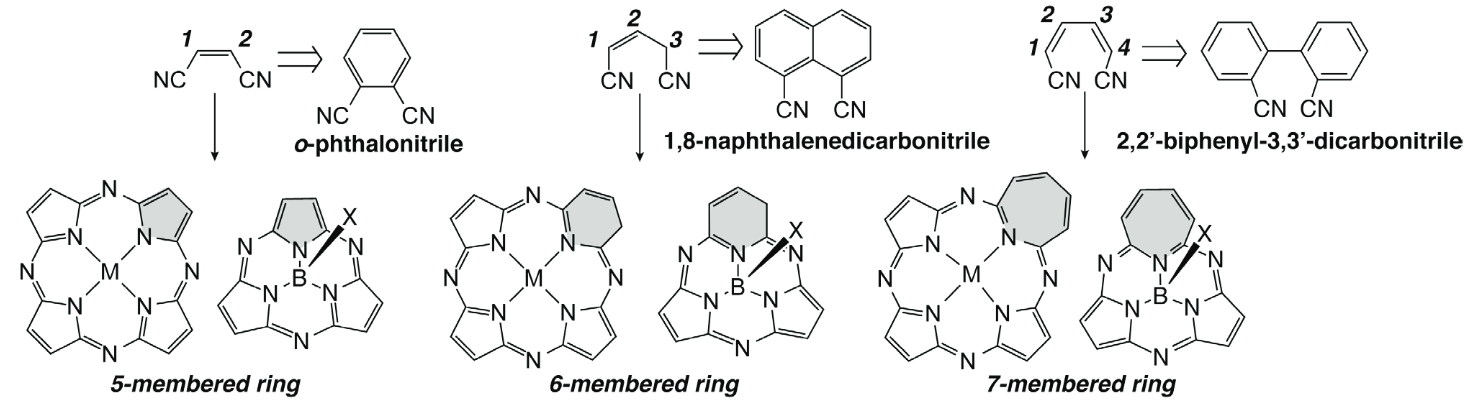
Shimizu, S.; Furuta, H.
Macroheterocycles2015, 54, 7323-7327 [Link]